Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (116 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

BOOK: Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine
6.99Mb size Format: txt, pdf, ePub
Initial treatment
• Resuscitation, control airway, monitor vital signs, fingerstick glucose, IV access
• Immobilization of C-spine if concern for cervical trauma
• Thiamine (100 mg IV)
prior to dextrose
to prevent exacerb. of Wernicke’s encephalopathy
• Dextrose (50 g IV push)
• Naloxone 0.01 mg/kg if opiates suspected; supportive care important in nearly all tox cases
• If concern for ↑ ICP ± herniation: ↑ head of bed; osmotherapy w/ mannitol or hypertonic saline; ↑ ventilation; dexamethasone for tumor edema; c/s neurosurgery (? decompress)
Diagnostic studies (
Continuum
2011;17:967)
• Labs: CBC, electrolytes, BUN/Cr, LFTs, NH
3
, tox screen, TSH, B
12
, ABG, U/A, ECG
• Imaging: head CT, consider MRI; radiographs to r/o C-spine fracture; CXR
• Lumbar puncture to r/o meningitis, SAH or noninfectious inflammation (eg, autoimmune)
• EEG to evaluate for nonconvulsive seizures, toxic/metabolic encephalopathy
Further treatment of delirium (
Annals
2011;154:746)
• Treat underlying acute illness, eliminate precipitating factors, provide supportive care
• Address sensory & cognitive impairments, increase familiarity
• Decrease/prevent infection/restraints if possible, remove lines/catheters if unnecessary
• Promote good sleep: reduce noise & night-time interventions; selective med if necessary
• Meds: consider antipsychotics, avoid benzos except for alcohol withdrawal or seizures
ANOXIC BRAIN INJURY
Prevalence (
NEJM
2012;367:1912)
• Pts with at least 5 min of cerebral hypoxia at risk • 1.5 million cardiac arrests per year in U.S.; for inPt arrest,
20% survival,
70% of Pts who survive will have a good long-term neurologic outcome
Initial evaluation (
Circulation
2010:S768)
• Neuro exam: arousal/verbal, eyes & other cranial nerves, motor response to pain • Imaging: usually not informative w/in first day after arrest, but should be done prior to initiating hypothermia if patient found down or has had head trauma
Induced hypothermia (
Circulation
2008;118:2452 & 2013;127:244)
• Indications: comatose (eg, no meaningful response to verbal stimuli) <6 h following cardiac arrest (not isolated resp. arrest). Fully studied only in VT/VF, but consider after asystole or PEA arrest or 6–12 h after cardiac arrest.
• Exclusion: pregnancy, CV instability despite pressors/assist devices, other cause of coma, persistent ↓ O
2
• Relative contraindications: major head trauma, coagulopathy/bleeding, major surgery <14 d, systemic infection/sepsis • Method: target temp 32–34°C × 24 h (from time of initiation of cooling). Can use cold saline infusions; ice packs to the head, neck and torso; cooling blankets; cooling vest or endovascular catheter if available. Goal to achieve target temp <6 h. Start rewarming 24 h after cooling is initiated (rewarm no faster than 0.5°C per h).
• Complications
cardiac dysrhythmias (bradycardia most common): if signif dysrhythmia or hemodynamic instability, d/c cooling and rewarm patient
coagulopathy: Pts can receive fibrinolytics, GP IIb/IIIa inhibitors, etc., and still undergo cooling. ✓ PT and PTT.
infection: ✓ surveillance blood cultures during cooling
hyperglycemia during cooling, hypoglycemia w/ rewarming; stop insulin if glc <200 mg/dL
hypokalemia during cooling, hyperkalemia w/ rewarming; keep K 4–5 mEq/L
Ongoing evaluation
• Neuro exam: daily focus on coma exam. No exam finding is reliable <24 h or on sedation. Pt needs to be off sedation for an adequate time to evaluate (depends on doses used, duration of Rx, metabolic processes in the individual Pt).
• Labs: daily CBC, PT/PTT, electrolytes. Serum neuron-specific enolase (NSE) on days 1–3
• Imaging: noncontrast CT 24 h after arrest; if unrevealing, consider MRI around days 3–5
• EEG: consider in all to exclude seizures or myoclonus; greatest risk during rewarming • Somatosensory evoked potentials (SSEP): helpful for prediction of poor outcome if cortical responses are absent bilaterally; perform 48 h after arrest (72 h if cooled)
Prognosis (
Neuro
2006;67:203;
NEJM
2009;361:605)
• Prior to cooling era, uniformly poor prognosis could be predicted at 72 h only in Pts who have absent pupillary and corneal reflexes, and no motor response to pain; or with absent SSEPs at 48 h. With cooling, it is less clear if the prior measures are as reliable.
• Otherwise, prognosis requires multifactorial approach considering exam, age, comorbid diseases, ancillary data (NSE, EEG, SSEP; imaging is less reliable for poor outcome) • When in doubt, err on the side of giving more time (esp. in younger Pts and induced hypothermia Pts)
SEIZURES
Definitions (
NEJM
2003;349:1257;
Epilepsia
2010;51:676)
•
Seizure
= abnormal, paroxysmal, excessive discharge of CNS neurons; occurs in 5–10% of the population; can range clinically from dramatic to subtle •
Epilepsy
= recurrent unprovoked seizures; 0.5–1.0% of population •
Generalized seizures
(involves brain diffusely)
Tonic-clonic
(grand mal): tonic phase (10–20 sec) with contraction of muscles (causing expiratory moan, cyanosis, pooling of secretions, tongue biting) → clonic phase (~30 sec) with intermittent relaxing and tensing of muscles
Absence
(petit mal): transient lapse of consciousness w/o loss of postural tone, usu pedi
Myoclonic
(infantile spasms & juvenile myoclonic epilepsy): sudden, brief contraction
•
Focal (partial) seizures
(involves discrete brain area, implies a structural lesion)
Simple
(w/o Δ MS) vs.
complex
(w/ Δ MS): motor, sensory and/or autonomic
Focal with secondary generalization:
starts focal, becomes generalized
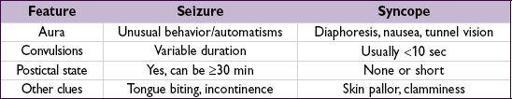
Differential diagnosis
•
Syncope
(
Lancet Neurol
2006;5:171)
•
Nonepileptic seizure
(NES, aka “psychogenic”): may see side-to-side head turning, asymmetric large-amplitude limb movements, diffuse shaking w/o LOC, and crying or talking during event • Other: metabolic disorders (eg, alcoholic blackouts, hypoglycemia), migraine, TIA, transient global amnesia, narcolepsy (cataplexy), nonepileptic myoclonus, tics, asterixis
Etiologies (varies strongly by age)
•
A
lcohol withdrawal, illicit drugs, meds (eg, β-lactams, bupropion, tramadol, metronidazole, meperidine, CsA, antidep., clozapine can lower seizure threshold) •
B
rain tumor or penetrating trauma •
C
erebrovascular disease, including subdural hematomas, hypertensive encephalopathy •
D
egenerative disorders of the CNS (eg, Alzheimer’s) •
E
lectrolyte (hyponatremia) & other metabolic (eg, uremia, liver failure, hypoglycemia) • Idiopathic (in ~60%)
Clinical manifestations
•
Aura
(sec to mins): premonition with paresthesias, focal motor contractions, abnormal smells/tastes, fear, depersonalization, déjà vu, autonomic changes, automatisms •
Ictal period
(sec to mins): tonic and/or clonic movements of head, eyes, trunk or extrem.
•
Postictal period
(mins to h): slowly resolving period of confusion, disorientation, and lethargy. May be accompanied by focal neurologic deficits (Todd’s paralysis).
•
Status epilepticus
: continuous tonic-clonic seizure ≥30 min or repeated seizures w/o resolution of postictal encephalopathy. Complications include neuronal death, rhabdomyolysis and lactic acidosis.
•
Nonconvulsive status epilepticus
: alteration of awareness (ranging from confusion to coma) w/o motor manifestations of seizure. Dx with EEG.
Clinical evaluation
• Seizure: patient usually w/o recollection, must talk to witnesses
unusual behavior before seizure (ie, an aura)
type & pattern of abnl movements, incl. head turning & eye deviation (gaze preference usually
away
from seizure focus)
loss of responsiveness
• HPI: recent illnesses/fevers, head trauma, sleep deprivation, medication compliance • PMH: prior seizures orFHx, prior meningitis/encephalitis, prior stroke or head trauma • Medications, alcohol and illicit drug use • General physical exam should include the skin, looking for neuroectodermal disorders (eg, neurofibromatosis, tuberous sclerosis) that are a/w seizures • Neurologic exam should look for focal abnormalities → underlying structural abnormality
Diagnostic studies (
Neurology
2007;69:1996)
• Laboratory: full electrolytes, BUN, Cr, glc, LFTs, tox screen, medication levels • EEG: during seizure can capture repetitive rhythmic activity (generalized seizures will typically have abnl EEG; partial may not); interictal EEG normal in 50% of Pts w/ epilepsy, and interictal epileptiform activity (spikes or sharp waves) seen in only 25% of Pts w/ epilepsy but up to 2% of normal population; sleep deprivation and repeated studies ↑ dx yield of EEG; video monitoring may help w/ nonepileptic seizures • MRI to r/o structural abnormalities; ↑ Se w/ fine coronal imaging of frontal & temporal lobes • LP (if no space-occupying lesion on imaging): if suspect meningitis (eg, fever, ↑ WBC, nuchal rigidity) or encephalitis and in
all
HIVPts
Treatment (
Lancet
2006;367:1087 & 2007;369:1000, 1016;
NEJM
2008;359:166)
Other books
The Troven (Kingdom of Denall Book 1) by Eric Buffington
Hopes by Linda Chapman
Mortal Gods by Kendare Blake
The Magician's Tower by Shawn Thomas Odyssey
Two To The Fifth by Anthony, Piers
Mistletoe & Kisses by Anthology
Martin Marten (9781466843691) by Doyle, Brian
Doctor Who: Ultimate Treasure by Bulis, Christopher
A Lantern in the Window by Bobby Hutchinson
The Secret in Their Eyes by Eduardo Sacheri