Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (70 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

Treatment
(
Blood
2011;117:3494)
• In absence of adverse prognostic factors (eg, anemia or sx) → no treatment • Allogeneic HSCT only potential cure → consider in young Pts with poor prognosis • Supportive care:
transfusions
; inconsistent benefit from androgens or Epo; splenectomy for blood counts refractory to transfusion or painful splenomegaly • Hydroxyurea for significant leukocytosis or thrombocytosis • Ruxolitinib (JAK1/JAK2 inhibitor) ↓ sx, ↓ splenomegaly, ↑ survival (
NEJM
2012;366:787 & 799) • Thalidomide and lenalidomide (improve red cell count)
Complications and prognosis
• Median survival
5 y; transformation into AML occurs at a rate of
8%/y • International Working Group (IWG) poor prognostic factors: age >65, WBC >25k, Hgb <10, blasts >1%,symptoms (
Blood
2009;113:2895). Stratification based on IWG factors allows prognostication at any point during clinical course (
Blood
2010;115:1703).
LEUKEMIA
ACUTE LEUKEMIA
Definition
• Clonal proliferation of hematopoietic progenitor with ↓ ability to differentiate into mature elements → ↑ blasts in bone marrow and periphery → ↓ RBCs, platelets and neutrophils
Epidemiology and risk factors
• Acute myelogenous leukemia (AML): ~14,000 cases/y; median age 66 y; >80% of adult acute leukemia cases • Acute lymphocytic leukemia (ALL): ~6000 cases/y; median age 14 y; bimodal with 2nd peak in adults • Risk factors:
radiation
,
chemo
(alkylating agents, topo II inhib), benzene, smoking • Acquired hematopoietic diseases: MDS, MPN (esp. CML), aplastic anemia, PNH
• Inherited: Down’s & Klinefelter’s, Fanconi’s anemia, Bloom syndrome, ataxia telangiectasia
Clinical manifestations
• Cytopenias →
fatigue
(anemia),
infection
(neutropenia),
bleeding
(thrombocytopenia) • More common in
AML
(esp. monocytic leukemias)
:
leukostasis
(when blast count >50,000/µL): occluded microcirculation → local hypoxemia and hemorrhage → dyspnea, hypoxia, headache, blurred vision, TIA/CVA; look for
hyperviscosity retinopathy
(vascular engorgement, exudates, hemorrhage)
DIC (esp. with APL)
leukemic infiltration of skin, gingiva (esp. with monocytic subtypes)
chloroma: extramedullary tumor of leukemic cells, virtually any location
• More common in
ALL
:
bone pain, lymphadenopathy, hepatosplenomegaly (also seen in monocytic AML)
CNS involvement (up to10%): cranial neuropathies, N/V, headache anterior mediastinal mass (esp. in T-cell); tumor lysis syndrome (qv)
Diagnostic evaluation
(
Blood
2009;114:937)
•
Peripheral smear
: anemia, thrombocytopenia, variable WBC (50% p/w ↑ WBC) + circulating
blasts
(seen in >95%; Auer Rods in AML) •
Auer Rods in AML) •
Bone marrow
: hypercellular with >20% blasts; cytogenetics, flow cytometry • Presence of certain
cytogenetic anomalies
, eg, t(15;17), t(8;21), inv(16) or t(16;16), are sufficient for dx of AML
regardless of the blast count
• ✓ for tumor lysis syndrome (rapid cell turnover): ↑ UA, ↑ LDH, ↑ K, ↑ PO
4
, ↓ Ca • Coagulation studies to r/o DIC: PT, PTT, fibrinogen, D-dimer, haptoglobin, bilirubin • LP (w/
co-admin of intrathecal chemotherapy
to avoid seeding CSF w/ circulating blasts) for Pts w/ ALL (CNS is sanctuary site) and for Pts w/ AML w/ CNS sx • TTE if prior cardiac history or before use of anthracyclines •
HLA typing
of Pt, siblings and parents for potential allogeneic HSCT candidates
ACUTE MYELOGENOUS LEUKEMIA (AML)
Classification
(FAB no longer used clinically;
Blood
2009;114:937)
• Features used to confirm myeloid lineage and subclassify AML to guide treatment: morphology:
blasts
, granules
granules
, ±
Auer rods
(eosinophilic needle-like inclusions) cytochemistry: myeloperoxidase
myeloperoxidase
and/or
nonspecific esterase
• Immunophenotype: myeloid antigens → CD13, CD33, CD117; monocytic antigens → CD11b, CD64, CD14, CD15
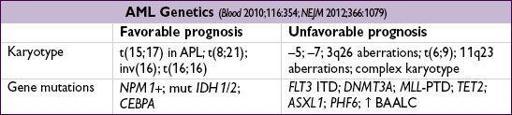
• Cytogenetics: important for prognosis. Intermed. risk = no favorable/unfavorable features.
Treatment
(
Blood
2010;115:453;
JNCCN
2012;10:984;
Lancet
2013;381:484)
• Induction chemo followed by consolidation Rx •
Induction chemo
: “7 + 3” = cytarabine × 7 d + ida/daunorubicin × 3 d. Cytarabine dose: continuous intermed. high dose (
high dose (
NEJM
2011;364:1027). Daunorubicin dose: age <60 → high (90 mg/m
2
); age
>
60 → standard (60 or 45 mg/m
2
) (
NEJM
2009;361:1249). Gemtuzumab ozogamicin (ɑ-CD33) ? benefit in fav/int risk AML (
Lancet
2012;379:1508) • ✓ for complete remission (CR) = normal peripheral counts, <5% BM blasts
CR
≠
cure
; ∴ must always f/u induction with
consolidation Rx
• If CR: consolidation Rx based on risk stratification (age, genetics, PS): chemo (eg, high dose cytarabine) if favorable risk;
CR: consolidation Rx based on risk stratification (age, genetics, PS): chemo (eg, high dose cytarabine) if favorable risk;
otherwise
→ allo-HSCT (
JAMA
2009;301:2349) • If CR: reinduction with similar chemotherapy (“5 + 2”) or alternative regimen • If relapse after CR: salvage chemo → allogeneic HSCT (↓ intensity conditioning if >60 y) • Supportive care: hydration + allopurinol or rasburicase for tumor lysis prophylaxis; transfusions; antibiotics for fever and neutropenia; antifungals for prolonged fever & neutropenia; hydroxyurea ± leukophoresis for leukostasis
CR: reinduction with similar chemotherapy (“5 + 2”) or alternative regimen • If relapse after CR: salvage chemo → allogeneic HSCT (↓ intensity conditioning if >60 y) • Supportive care: hydration + allopurinol or rasburicase for tumor lysis prophylaxis; transfusions; antibiotics for fever and neutropenia; antifungals for prolonged fever & neutropenia; hydroxyurea ± leukophoresis for leukostasis
Prognosis