Junk DNA: A Journey Through the Dark Matter of the Genome (37 page)
Read Junk DNA: A Journey Through the Dark Matter of the Genome Online
Authors: Nessa Carey

When scientists knocked out the scissors enzyme in all tissues of adult mice, they found defects in the bone marrow, but also in the spleen and the thymus. All three of these tissues produce cells required for fighting infection and were expected to have a large population of stem cells. This finding was consistent with the smallRNA systems having a role in stem cell control. The mice all died, but this was due to a massive deterioration of their intestinal tracts. This is also consistent with a role in stem cells. Our intestines are constantly losing cells that are sloughed off during the continuing activity of the digestive system. These cells have to be replaced every day so we would expect there to be a very active stem cell population.
13
However, it wasn’t clear exactly how
the loss of the scissors enzyme resulted in dramatic damage to the intestines, although it may have been related to abnormalities in the way the mice processed fats in their diet.
These effects were very dramatic, but that doesn’t mean that these are the only tissues where smallRNAs play an important part. Because the mice died relatively quickly, this may have masked more subtle symptoms in other tissues. In order to investigate this, researchers can use a more discriminating version of the adult knockout technique. With this amended technology, they are able to inactivate the scissors gene in selected tissue types in adult mice.
Many of the results were entirely consistent with an impact on stem cell populations. For example, when the scissors gene was inactivated in the cells of the hair follicle in adult mice, fur didn’t grow back properly after plucking.
14
It would be tempting to speculate from these results that the smallRNA networks are required to keep stem cells doing their job of replenishing specialised cells. But this is too simplistic. Just as we all strive to make our salary last until the next payday, our bodies need to make sure they don’t use up their stem cells too quickly. They are precious, and when they’re gone, they’re gone. Once we appreciate that, it seems obvious that some smallRNA networks are required to stop stem cells from irreversibly converting into mature tissue cells. There is actually a balance that needs to be struck, and this is shown in Figure 18.2.
The skeletal muscles contain stem cells,
b
and it’s worth keeping these quiescent most of the time so that they don’t get used up too early. This exhaustion of the stem cell reservoir is partly responsible for some of the muscle loss we have encountered already in conditions such as Duchenne muscular dystrophy. There are proteins in muscle stem cells that normally stop them from converting into mature muscle cells. However, if there is an acute injury in
healthy individuals, or loss of muscle cells in a dystrophic condition, these proteins are down-regulated. This is achieved at least in part by switching on expression of specific smallRNAs. The smallRNAs bind to the messenger RNAs that carry the code for these proteins, and less protein is produced. The brakes are taken off the stem cells and they convert into mature muscle.
15
,
16
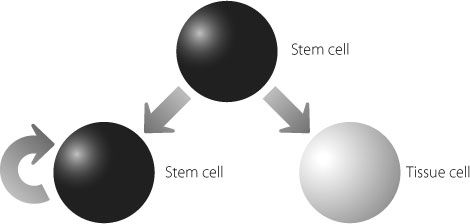
Figure 18.2
When a stem cell divides it can create either another stem cell, which can also keep dividing, or a differentiated cell that will not create more stem cells.
A similar effect can be seen in the heart. The adult cardiac muscle does contain some stem cells, although they aren’t huge in number and they are hard to convert into mature heart tissue. This is one of the reasons why heart attacks are so damaging. In a heart attack, cardiac muscle dies and our bodies find it very difficult to create replacement tissue. Instead, we get scarring on the heart and the organ doesn’t work properly. This leads to the long-term difficulties many heart attack survivors encounter, and is why in some cases they never regain full health.
Although it might seem that it would be great to be able to activate cardiac stem cells to produce new muscle, experiments from mice suggest that the situation isn’t straightforward. It would seem that in the heart the smallRNAs prevent stem cells converting into cardiac muscle. If the scissors enzyme that produces
smallRNAs is switched off in an adult heart, the heart begins to grow. Unfortunately, it does so in a way that is potentially damaging, resulting in a condition known as cardiac hypertrophy. This is unlike the helpfully strong heart muscle of elite athletes. Instead it is more like the abnormal thickening of the heart walls found in people with high blood pressure. Its seems to happen because loss of scissors activity causes the stem cells to stop acting like adult cells and drives a gene expression pattern that’s more like the one seen during development.
17
It might seem odd that reactivating cardiac stem cells isn’t necessarily helpful but perhaps it’s a trade-off. In evolutionary terms, the most important consideration for animals is to live long enough to reproduce and pass on one’s genetic material. The control of cardiac development is geared towards making sure that our hearts are good enough to get us to this point. From an evolutionary perspective, it doesn’t really matter if this means that when we are older we can’t repair our hearts. This is a problem for humans because we like living longer than evolution deems strictly necessary.
SmallRNAs and the brain
Although we usually think of our brains as being fully formed in adults, recent data have shown that even in this organ there are some stem cells. In animals that rely on a highly developed sense of smell, these stem cells can be activated to form neurons that respond to new scents. This allows the animal to tailor the smells to which it responds most strongly. A protein in the stem cells drives them into differentiating into a specific type of responsive neuron. Expression of this protein is usually held in check by a smallRNA. When researchers inhibited the expression of this smallRNA in mice, the protein was up-regulated and the neural stem cells differentiated into neurons associated with detection of smell.
18
The suspicion is that the smallRNA is down-regulated naturally when
the mouse smells something new, although the signalling pathways that drive this repression haven’t been identified yet.
SmallRNAs are involved in everyday cellular activities, fine-tuning responses to constantly fluctuating environments. It can be difficult to unravel how this fine-tuning operates, because each individual smallRNA has a relatively small effect. It’s the overall cumulative effect of multiple smallRNAs acting in vast but subtle networks that is their most important feature. Even so, enough intriguing data are emerging to give us confidence that this class of miniature junk minions has real impact.
The brain appears particularly sensitive to perturbations of the smallRNA landscape. The impact of such changes varies depending on the regions of the brain involved, but also on the timing of the perturbations. This in turn probably reflects the importance of cross-talk between all the different smallRNAs and all the other messenger RNAs and proteins whose expression is tightly controlled in the brain.
A striking example of this is found when the scissors enzyme is inactivated in a region called the forebrain in adult mice.
19
The expression of smallRNAs is lost, and at first it seems like this is quite a good thing for the animals. For about three months the mice are smarter than usual. They perform better at tests, whether these are based on fear or on reward. Their memory skills are significantly improved. But in case anyone is thinking of trying this at home on their own brain (everyone is very exam-focused these days), there is a downside. The intellectual star of these smart mice shone brightly, but it didn’t shine for long. About twelve weeks after the scissors enzyme was inactivated, the brains of the furry little geeks began to degenerate.
This delayed reaction was also found in another situation where smallRNAs were shown to be important in the brain. This may imply that smallRNAs are fairly stable in brain cells, and take a while to die down. The scissors enzyme was inactivated in brain
cells of two-week-old mice, in a region that is involved in the control of movement. As expected, this resulted in a major drop in the expression of smallRNAs. The mice appeared fine at first but eleven weeks later they began to develop movement problems. Analyses of their brains showed that the neurons that lacked the ability to make smallRNAs had died.
20
SmallRNAs can turn up in all sorts of unexpected situations. One of the targets for alcohol in our brains is a protein that regulates how signals pass across the membranes of cells.
c
The messenger RNA for this protein can occur in lots of different versions, depending on how the amino acid-coding regions are spliced together. Alcohol induces the expression of a particular smallRNA which can bind to the untranslated region at the end of some of these variant messenger RNAs. This leads to selective destruction of the messenger RNAs that code for some variants of the proteins, but not others. This change in the population of the possible proteins leads to a skewing in the responses of the neurons to alcohol, and is an important part of the tolerance to alcohol that is a component of addiction.
21
This mechanism is summarised in Figure 18.3. SmallRNAs have also been implicated in addictive responses to other drugs, such as cocaine.
22
SmallRNAs and cancer
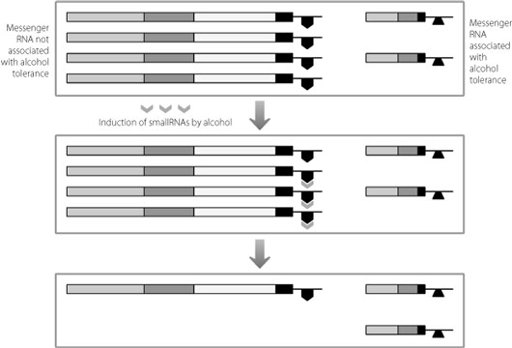
Figure 18.3
SmallRNAs induced by alcohol can bind to messenger RNAs that don’t create alcohol tolerance. The smallRNAs don’t bind to the messenger RNA molecules that promote alcohol tolerance. This leads to a relative preponderance of the messenger RNA molecules that code for protein versions associated with tolerance to alcohol.
Mis-expression of smallRNAs has been implicated in a number of diseases that have a major impact on global human health. These include cardiovascular diseases
23
and cancer.
24
The latter is perhaps unsurprising, given that cancer represents abnormalities in cell fate and cell development, and smallRNAs are very important in these processes. One very clear example of the importance of smallRNAs in cancer is in a type of tumour that is characterised
by inappropriately expressing developmental rather than postnatal genes. It’s a subtype of a childhood brain tumour which usually presents before the age of two. Sadly, it’s a very aggressive form of cancer, and the prognosis is poor even with powerful therapy.
d
The cancer develops following an inappropriate rearrangement of genetic material in the brain cells. A promoter that normally drives strong expression of a protein-coding gene recombines with a particular smallRNA cluster. This whole rearranged region is then amplified, meaning multiple copies are produced in the genome. As a consequence, the smallRNAs downstream of the
relocated promoter are expressed far too strongly. The levels of the smallRNAs are between 150 and 1,000 times higher than they should be.
The cluster codes for over 40 different smallRNAs, and is in fact the largest cluster in primates. It is usually only expressed early in human development, in the first eight weeks of foetal life. Switching it on strongly in the brain of an infant has a catastrophic effect on gene expression. One of the downstream effects of this is to drive expression of an epigenetic protein which adds modifications to DNA. This leads to global changes in DNA methylation patterns, resulting in abnormal expression of a whole range of genes, many of which should be expressed only when the immature brain cells are dividing during development. This generates a cancerous cell programme in the infant.
25
