Junk DNA: A Journey Through the Dark Matter of the Genome (36 page)
Read Junk DNA: A Journey Through the Dark Matter of the Genome Online
Authors: Nessa Carey

These data showed that, over time, even the boys who received the drug began to decline (look at the difference between 24 and 48 weeks), but this decline was dramatically slower than when the condition was running its normal course.
The results from this trial caused enormous excitement. Finally, it looked like there might be hope developing in the treatment of a previously intractable disorder. Even if the treatment didn’t cure the patients, it might significantly slow down the development of the irreversible symptoms. This was what everyone researching in the field, and the families of affected boys, had been working towards for decades. True, it wouldn’t work for all Duchenne sufferers, but between 10 and 15 per cent of patients were expected to be eligible for this approach, based on the kind of mutation in their dystrophin gene.
Just six months later, those hopes were in tatters. GlaxoSmithKline ran a larger trial and this time couldn’t find any significant difference between the treated and untreated groups.
22
The results from larger trials are more reliable than ones from smaller studies because they are less likely to be affected by odd patterns that look like a response but aren’t. GlaxoSmithKline had no doubts about its large trial, convinced that if there had been a genuine effect of the drug, it would have been detected. They
handed the drug back to Prosensa and walked away. Prosensa is continuing with clinical studies, although its share price tanked after GlaxoSmithKline departed, reflecting concerns by analysts that this programme may be doomed.
There is another company that is also trying to exploit splicing patterns to leap over the troublesome region in the dystrophin gene in the same patient groups. This company is called Sarepta, and it is using a similar approach to treating the affected boys. Although the company remains very upbeat about its programme, the Food and Drug Administration has questioned whether its trials are large enough to give genuinely conclusive results. For example, one of the studies in which a dramatic difference between the untreated and treated groups was seen only contained twelve patients.
Investors in the companies are no doubt feeling a chill breeze, but it can’t begin to compare with what the families of affected boys must have gone through and be going through every day.
It would be tempting to look at the science in this chapter and decide that splicing is more trouble than it’s worth. It certainly seems to be an example of Sod’s law – if something can go wrong, it will. But the reality is that the same is true of almost every biological process. Billions of bases, thousands of genes, trillions of cells, billions of people. It’s a numbers game; nothing goes right every time. But the fact that this process of joining together split genes has been maintained through hundreds of millions of years of evolutionary history, using a highly conserved system, makes it pretty clear that the advantages of the sophistication, additional information content and sheer flexibility more than compensate for the off days.
Footnotes
a
If you haven’t already, go and see
The Lego Movie
, it’s excellent.
b
As a reminder, the junk regions between amino acid-coding regions are known as introns. The amino acid-coding parts themselves are known as exons.
18. Mini Can Be Mighty
Perhaps because we are quite large animals, we tend to be most impressed by other large animals. And that’s OK. After all, a big cat such as a jaguar is an impressive creature. We also tend to be impressed because the jaguar is a hunter, a top carnivore. An ant, by comparison, looks rather puny, even if it’s one of the Central and South America species of army ant. Sure, there is a certain gory charm in an insect with jaws so large and strong you can use them to hold the sides of a wound together. But it’s still difficult to be frightened by something we can squash with a small downward stomp of a hiking-booted foot.
But a colony of army ants, well that’s a different matter. A colony probably eats as much flesh as a jaguar does. If you saw a column of them heading your way you might be tempted to put on your boots and run like hell, rather than indulging in a cheery ant-stomping dance.
And so it is with our genome. There are thousands of examples of a particular type of very small junk nucleic acid.
1
Each one plays a role in fine-tuning gene expression, and individually their effects are subtle. But when we look at the totality of their impact, they are an impressive horde.
Welcome to the world of smallRNAs, the mighty army ants of our genome. As their name suggests these RNA molecules are little, typically just 20 to 23 bases in length. We can think of them as nudging molecules, which impart an additional fine-tuning process to control of gene expression.
Figure 18.1 shows how these smallRNAs are produced, and
how they work. They are generated from double-stranded RNA molecules. They then bind to the untranslated regions at the ends of messenger RNAs, to create a new double-stranded RNA. The creation of this double-stranded structure, dependent on the interaction of one junk sequence with another, has one of two effects on the messenger RNA. It can target the messenger RNA for destruction, or it can make it difficult for the ribosomes to translate the messenger RNA sequence into proteins. The end result is essentially similar, a drop in the amount of protein generated from that specific messenger RNA.
a
2
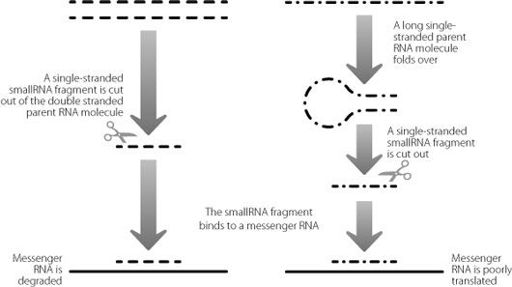
Figure 18.1
Schematic describing how the cell creates two different classes of smallRNAs from longer RNA molecules. The two classes repress gene expression in different ways, as shown at the bottom of the illustration.
The smallRNAs that trigger the destruction of messenger RNA molecules have to be a perfect match for their targets. The ones that inhibit the translation of the messenger RNAs are much more promiscuous. They will bind to a messenger RNA even if only a seed sequence of six to eight consecutive bases matches the target. One of the consequences of this is that a single smallRNA may bind to more than one type of messenger RNA, and slow down its translation. Another potential consequence is that the relative levels of the different messenger RNAs in a cell will influence the extent to which each is controlled by a particular smallRNA. This means that any given smallRNA will have a different effect depending on which of its targets is being expressed in a cell, and the ratios of the target molecules.
SmallRNAs – for good, for bad
There is a single cluster of smallRNA molecules that plays an important role in the regulation of a select cell type in the immune system. If this cluster of smallRNAs is over-expressed in mice, the animals develop a fatal over-activation of the immune system.
3
,
4
On the other hand, mice that lack this cluster altogether die around the time of birth. In humans, the loss of one copy of this cluster leads to some cases of a rare condition called Feingold syndrome.
5
Patients with this disorder have variable symptoms, often including malformations of the skeleton, kidney problems, gut blockages and moderate learning disabilities.
6
The consequences of disrupted expression of this cluster of just six smallRNAs seem puzzlingly diverse. But perhaps this isn’t so surprising, as researchers have calculated that this cluster alone may target over 1,000 protein-coding genes.
7
The junk sequences that code for smallRNAs are often located within other junk regions, such as the genes producing the long non-coding RNAs.
8
There is a condition called human
cartilage-hair hypoplasia, which was originally identified in an Amish community, where one in ten of the community is a carrier of the causative mutation. This is an incredibly high carrier frequency and almost certainly reflects the fact that this community was originally founded by just a small number of families. The affected children have defects in the formation of their skeletons, resulting in a short-limbed form of dwarfism, and light hair that is fine but sparse. The patients also tend to have a variable range of other defects.
The mutations that cause this condition lie in a long non-coding RNA gene. But this long gene encompasses two smallRNA genes, junk within junk, and many of the mutations affect the smaller moieties. The changes disrupt the structures of the smallRNAs so that they aren’t processed properly by the cutting enzyme represented by scissors in Figure 18.1. As a consequence, they aren’t expressed at their normal level. Between them, these two smallRNAs regulate over 900 protein-coding genes. These include genes known to be involved in skeletal and hair development, but also in a number of other systems. This is presumably why mutations that affect the levels and functions of these smallRNAs can also lead to problems in a range of organ systems in the affected children.
9
Given how important smallRNAs are for fine-tuning of gene expression, it’s perhaps not surprising to learn that these junk molecules have a major role during development. This is the stage in life where apparently minor fluctuations in gene expression can have a significant impact (remember our Slinky falling down the stairs?).
SmallRNAs and stem cells
A beautiful example of the importance of smallRNAs comes from reprogramming human tissue cells to become pluripotent stem cells, potentially capable of building any tissues we need. This is
the technology that we first met in Chapter 12, and which is shown in Figure 12.1 (
page 165
). Although the original work for which the Nobel Prize was awarded so quickly was extraordinary, it had some limitations. Although the master regulator proteins could push the developmental Slinky back up a flight of stairs, they did so fairly inefficiently. Only a tiny percentage of cells were converted, and the process took many weeks. Five years after those ground-breaking findings, other researchers extended this work. They treated the adult cells with the same master regulators used in the original experiments. But they also added something else. They over-expressed a cluster of smallRNAs which had been shown to be highly expressed in normal embryonic stem cells. The scientists found that when they over-expressed these smallRNAs along with the original master regulators, adult cells changed back to pluripotent stem cells, as we would expect. But the percentage of cells that converted to stem cells was more than a hundred times greater than with just the master regulators alone. The process also happened much more quickly. Conversely, if they used the master regulators but knocked down the expression of the endogenous smallRNA cluster in the adult cells, the reprogramming efficiency dropped dramatically. This demonstrated that this particular cluster of smallRNAs does indeed play a critical role in helping to regulate the signalling networks that control cell identity.
10
,
11
Adult tissues also contain stem cells. These are able to create cells for their specific tissues, rather than multiple cell types. These are important for growth as we move from baby to adult, and also for repairing wear and tear. Some tissues retain a very active stem cell population even late into life. A classic example would be the bone marrow, which keeps producing the cells we need to fight infection and to patrol against potentially cancerous cells. One of the reasons the very elderly are particularly prone to infections and cancer is because their bone marrow stem cells eventually run out, leaving them with holes in their immune barricades.
There are data showing that stem cells and adult cells from human tissues express different patterns of smallRNAs. But expression data are always difficult to interpret, because of the cause-or-effect problem. Are the different patterns of smallRNAs driving the differences in cell activity and function, or are they simply a bystander consequence of the cellular changes? The fact that predicted sequence pairings between individual smallRNAs and the untranslated regions of at least half of all messenger RNA molecules have been preserved through evolution suggests a causal effect.
12
But to address this question more directly, scientists have frequently turned to our close cousin, the mouse.
Researchers have found ways of knocking out genes only in adult tissues, which has created a very powerful tool set for investigations. This handy technique means that mice develop in the usual way, so we don’t need to worry that symptoms are caused by pathways and networks going wrong during development. This approach has been used to work out what happens if the enzyme that is required to produce smallRNAs (the scissors in Figure 18.1) is inactivated in adult cells. This will interfere with production of all smallRNAs and so show us where they play an important role. It won’t, however, tell us exactly which smallRNAs are involved.
