Read Junk DNA: A Journey Through the Dark Matter of the Genome Online
Authors: Nessa Carey
Junk DNA: A Journey Through the Dark Matter of the Genome (38 page)

This cross-talk between smallRNAs and the epigenetic machinery of the cell may be significant in other situations where cells become predisposed to cancer. This mechanism can amplify the impact of disrupted smallRNA expression, by altering epigenetic modifications, which can be passed on to daughter cells. This can start a hard-wiring in of potentially dangerous alterations in gene expression.
Not all the steps have been unravelled in how smallRNAs interact with epigenetic processes, but hints are emerging. For example, a particular class of smallRNAs which trigger increased aggressiveness in breast cancer targets the messenger RNAs for certain enzymes that remove key epigenetic modifications. This alters the pattern of epigenetic modifications in the cancer cell, and further disrupts gene expression.
26
Many cancers are surprisingly difficult to monitor in a patient. They may be inaccessible, so that they are hard to sample. This can make it difficult for clinicians to monitor how a cancer is changing, and exactly how it is responding to therapies. They may have to rely on indirect measures, such as imaging the tumour on a scan.
Some researchers have suggested that smallRNA molecules may provide a new technique for following the natural history of a tumour. When cancer cells die, this often results in the smallRNAs leaving the cell as it breaks down. These little junk molecules are often complexed with cellular proteins, or wrapped in fragments of the cell’s membranes. This makes them very stable in body fluids, so they can be isolated and analysed. Because the amounts are low, researchers need to use very sensitive analytical techniques. This isn’t impossible though, because nucleic acid sequencing sensitivity is improving all the time.
27
Data in support of this approach have been published for breast
28
and ovarian cancer,
29
among others. In the case of lung cancer, circulating smallRNAs have been analysed and shown to be useful at discriminating between patients with a solitary lung nodule that is benign (doesn’t require therapy) from patients where the nodule is a tumour (and needs treatment).
30
Dead horses and silenced genes
SmallRNAS are turning up in all sorts of unexpected situations. There is a really horrible viral infection called North American eastern equine encephalitis virus. It’s transmitted by mosquito bites. When this virus infects horses, the animals die. The situation isn’t much better in humans, where the fatality rate is between 30 and 70 per cent. The patients die because the virus gets into the central nervous system and causes severe inflammation of the membranes around the brain.
31
The virus that causes the infection has a genome that is made of RNA, not DNA.
When this virus first enters the human bloodstream following a mosquito bite, it is taken up by white blood cells. These are the front line in surveillance against invaders. But then something very odd happens. A smallRNA naturally produced by the white blood cells binds to the end of the virus’s RNA genome, and stops it from coding for protein.
This might seem like a good thing but it’s quite the opposite. Our white blood cells normally recognise if they have been infected by a virus. The cells will initiate a set of reactions including raising body temperature, and producing various anti-viral chemicals. Together, these repel the tiny invaders.
But when the smallRNA in the white blood cells binds to the equine encephalitis virus genome, the virus goes quiet. Consequently, the immune system doesn’t notice that the body has been infiltrated. This leaves other viral particles free to drift through the body. If some of them reach the central nervous system, they can then trigger the lethal responses in the brain tissues.
32
The researchers described this in terms of the virus hijacking the smallRNA system, and it doesn’t seem to be the only example of this happening. The hepatitis C virus also has an RNA genome. When this virus infects liver cells, the viral RNA binds to a small-RNA naturally expressed by these cells. In this case, the binding stabilises the viral genome, making it harder to break down. As a consequence, more viral proteins are produced, and the infection becomes more damaging and more aggressive.
33
It’s pretty clear that smallRNAs are involved in a whole range of human pathologies from infection to cancer, and from development to neurodegeneration. This of course raises an interesting question: if junk DNA can cause or contribute to disease, is it also possible to use junk to fight common human illnesses?
Footnotes
a
The type of smallRNA that triggers degradation is called microRNA, or miRNA. The type that triggers poor translation is called small interfering RNA, or siRNA. In order to avoid excessive technical language, the term smallRNA will be used to describe both of these.
b
These are known as satellite cells
c
This is a protein called BK, which is a potassium channel.
d
These are known as supratentorial neuroectodermal tumours.
19. The Drugs Do Work (Sometimes)
Billions of dollars are spent every year by companies trying to create new drugs to treat human diseases. They hope to find ways to tackle unmet medical needs, a situation that is becoming ever more urgent with the increasing age profile of the global population. The breakthroughs in the understanding of the impact of junk DNA on gene expression and disease progression are triggering a slew of new companies seeking to exploit this field. Specifically, most of the new efforts are in using non-protein-coding RNAs as drugs in themselves. The basic premise is that junk RNA – long non-coding, smallRNAs or another form called antisense – will be given to patients, to influence gene expression and control or cure disease.
This is very different from the way we treat diseases at the moment. Historically, most drugs have been of a type known as small molecules. These are chemically created and are relatively simple in shape. Examples of some common small molecule drugs are shown in Figure 19.1.
More recently, we have learnt how to use proteins as drugs. Probably the most famous is insulin, the hormone that diabetics use to regulate their blood sugar levels. Antibodies are another very successful type of protein drug. These are engineered versions of the molecules we all produce to fight infections. Drug companies have found ways of adapting these so that they will bind to over-expressed proteins and neutralise their activities. The bestselling antibody is one that treats rheumatoid arthritis very effectively, but
there are others that treat conditions as diverse as breast cancer and blindness.
1
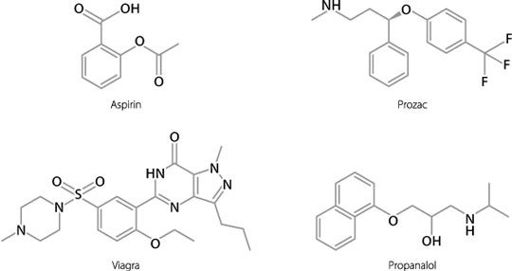
Figure 19.1
Structures of some commonly used small molecule drugs.
Small molecules and antibodies have advantages and drawbacks. Small molecules are usually relatively cheap to synthesise and easy to administer, frequently just needing to be swallowed. Their drawback is that they don’t last very long in the body, which is why we need to take them on a regular basis. Antibodies can last for weeks or even months in the body, but they have to be injected by a medical professional, and they are very expensive to manufacture.
There are some other drawbacks too. Antibodies are only effective against molecules that are in body fluids such as blood, or are on the surface of cells. These drugs can’t get inside cells to do their work. Depending on their structure, small molecules can get inside cells if necessary. But they may be limited in the kinds of proteins that they can control.
Small molecules act like a key in a lock. If you are inside your house, the easiest way to stop someone coming in is to lock your
door and leave the key in it. If you wanted to stop anyone else from ever entering, you could even lock the door using a slightly defective key, which jams the lock for ever.
This works because the key fits into the lock really snugly. But what you can’t do is use a key to block one of those old-fashioned external sliding bolts. There is nowhere for the key to fit in this, it will just keep slipping around on the surface. This is also true of our cells. There are lots of proteins inside our cells that we would like to control but we can’t create small molecules against them, because of the protein structure. They don’t have nice neat clefts or pockets that we can fit drugs into. Instead, they have large flat surfaces, and there is nowhere for a small molecule to lodge.
We could try to make bigger molecules that can cover the whole flat surface. The problem with this is that once we get above a certain size with our drugs they don’t circulate well around the body, and they can’t get into the cells to do their job.
There’s also another problem. It’s hard enough to create drugs that will successfully get inside cells, bind to a specific protein, and stop that protein from working. But it’s incredibly difficult to create drugs that will get inside cells, bind to a specific protein and then make that protein work harder, or faster, or better. And it’s practically impossible to make traditional drugs that will drive up the expression of one specific protein, or switch on one and only one gene.
Could junk DNA save us?
This is why there is so much interest in finding new approaches to drug therapies, and why the increasing knowledge of junk DNA is so important. By using long non-coding RNAs or smallRNAs, it is theoretically possible to target pathways that can’t be tackled using traditional small molecule or antibody drugs. It won’t matter that the targets are inside cells and have large flat surfaces. It won’t
matter that we need to increase expression or activity of a protein or gene. We can use this new approach to tackle any type of target.
Theoretically.
That’s the word to focus on. Theoretically. Ideas are common, success is rare. So it’s worth taking a good look at where the reality is before we all cash in our pensions to invest in the latest biotech company working in this space. There is a lot of activity going on,
2
so it’s worth concentrating our analysis on a few leading examples.
There is a protein produced in the liver that is responsible for transporting some other molecules around the body. Globally, there are about 50,000 people who have inherited a mutation in the gene that produces this protein. There are lots of different mutations, but they all seem to have a similar effect. They all change the activity of the protein so that it starts to transport the wrong molecules.
a
3
When this happens, deposits, which include a mixture of normal and mutated protein, begin to build up in tissues. Patients may have a range of symptoms, depending on the tissues in which the deposits build up. In about 80 per cent of known cases, the heart is the main organ that is affected, and this leads to potentially lethal cardiac defects. In many of the other 20 per cent of cases, the deposits build up in the nerves and spinal cord. This can lead to debilitating problems with a range of organs, including abnormal and painful sensory responses to mild stimuli.
A company called Alnylam has created a smallRNA, attached to sugar molecules, which can be injected into patients. The small-RNA binds to the untranslated region at the end of the messenger RNA that codes for the protein that is mutated in this disease. This targets the messenger RNA for destruction.
In 2013 the company released data from a phase II clinical study of their drug. They found that when they injected the drug
into patients, there was a rapid and sustained drop in the circulating levels of the mutated and normal versions of the protein.
4
This is encouraging, but not yet a cure. The assumption is that a drop in circulating levels will lead to a slowdown in the build-up of tissue deposits. This in turn should lead to at least a slowdown in the progress of the disease. But we won’t know if that is the case until a bigger trial is carried out, in which the actual symptoms and disease progression are monitored. Only if the drug impacts on these will it be considered a success.
A different company, called Mirna Therapeutics, has created a smallRNA which mimics one known to be important in cancer. The endogenous smallRNA is a tumour suppressor, and its overall effect is to hold back cell proliferation. It does this by negatively regulating the expression of at least twenty other genes that try to push the cells into division. Expression of this smallRNA is often lost or decreased in cancer patients, removing the brakes on cell division. The hope is that by reintroducing it into cells, the normal pattern of gene regulation will be restored, and the cells will stop proliferating so quickly.

