Why Beauty is Truth (53 page)
Read Why Beauty is Truth Online
Authors: Ian Stewart

At first sight, each force bears little resemblance to the others. But beneath the surface there are signs that these differences are less important than they seem. Physicists have teased out evidence of a deeper unity, suggesting that all four forces have a common explanation.
We feel the consequences of gravity all the time. When we drop a plate and it shatters on the kitchen floor, we see gravity pulling it towards the Earth's center and the floor getting in the way. The plastic pigs on the freezer door (well, that's what you will find in
our
house) remain in place thanks to the magnetic force, which Maxwell showed was merely one aspect of the unified electromagnetic force. The electrical aspect runs the freezer. Less obviously, the shattering plate also reveals the consequences of the electromagnetic force, because this is the main force acting in chemical bonds to hold bulk matter together. When the stress on the plate becomes too great for the electromagnetic force to hold its molecules together, it breaks.
The two remaining forces, which act on the level of the atomic nucleus, are not so readily apparent; but without them there would not be any matter at all, because they hold atoms together. They are why the plate, pigs, freezer, floor, and kitchen exist.
Other types of force could in principle give rise to other types of universe, and our ignorance of such possibilities is almost total. It is often claimed that without the particular forces we have, life would be impossible, proving that our universe is amazingly finely tuned to make life possible. This argument is bogus, a wild exaggeration based on too limited a view of what constitutes life. Life
like ours
would be impossibleâbut it is the height of arrogance to assume that our kind of life is the only kind of organized complexity that could exist. The fallacy here is to confuse
sufficient
conditions for life (those aspects of our universe on which our kind of life depends) with necessary ones.
The first of the four forces to be formulated scientifically was gravity. As Newton observed, this is an attractive force: any two particles in the universe, he said, attract each other gravitationally. The force of gravity is long-range: it falls off fairly slowly with distance. On the other hand, the gravitational force is much weaker than the other three: a tiny magnet can attach a plastic pig firmly to the fridge, even though the entire Earth is trying to pull it off through the force of gravity.
The next fundamental force to be isolated was electromagnetism, under whose influence particles may either attract or repel each other. What distinguishes the two is whether the particles have the same electric charge or the same magnetic polarity. If they do, the force is repulsive; if not, it is attractive. Again, this force is long-range.
The nucleus of an atom is assembled from smaller particlesâprotons and neutrons. Neutrons, as the name suggests, have no electric charge, but all protons have positive charge. The electromagnetic repulsion among protons should cause the nucleus to explode. What holds it together? Gravity is too weakâthink of the plastic pigs. There must be some other forceâwhich physicists labeled the strong nuclear force.
But if the strong force can overcome electric repulsion, why don't all of the protons in the universe get sucked together into one gigantic atomic nucleus? Clearly, the effect of the strong force must fall off rapidly at distances greater than the size of the nucleus. So the strong force is short-range.
The strong force does not explain the phenomenon of radioactive decay, in which atoms of certain elements “spit out” particles and radiation, and change to different elements. Uranium, for example, is radioactive and eventually turns into lead. So there must be yet another subatomic force. This is the weak force, and it is even shorter-range than the strong force: it acts only at a distance one-thousandth the size of a proton.
Physics was a lot easier when the only building blocks of matter were protons, neutrons, and electrons. These “elementary particles” were the components of the atomâwhich, it transpired,
did
split, even though the name means “indivisible.” In Niels Bohr's early model, the atom was visualized as a tight collection of protons and neutrons orbited by much lighter, distant electrons. The proton carried a fixed positive electric charge, the electron carried the same amount of charge but negative, and the neutron was electrically neutral.
Later, as quantum theory developed, this solar-system image was replaced by a subtler one. The electrons didn't orbit the nucleus as well-defined particles but kind of smeared themselves around it in interestingly shaped clouds. These clouds were best interpreted as clouds of probability. If you looked for an electron, you were more likely to find it in the cloud's denser regions and less likely to find it in the sparse regions.
Physicists invented new ways to probe the atom, break it into pieces, and probe the inner structure of those pieces. The main method, still in use, is to hit it with another atom or particle and watch what flies off. Over timeâthe story is too complicated to tell in detailâmore and more different kinds of particle were found. There was the neutrino, which could
pass through a million miles of lead unhindered and was therefore rather hard to detect. There was the positron, like an electron but with the opposite electrical charge, predicted by Dirac's matter/antimatter symmetry.
As the number of “elementary” particles grew to more than sixty, physicists began to seek deeper ordering principles. There were too many building blocks for them to be fundamental. Each type of particle could be characterized by a series of properties: mass, charge, something called “spin” because the particles behaved as though they were spinning around some axis (except that this was an outmoded image and whatever spin was, it wasn't really that). The particles did not spin in space, like the Earth or a spinning top. They “spun”âwhatever that meantâin more exotic dimensions.
Like everything in the quantum world, most of these features came in integer multiples of basic, very tiny amountsâquanta. All electrical charges were integer multiples of the charge on a proton. All spins were integer multiples of the spin of an electron. It was not clear whether mass was similarly quantized; the masses of the fundamental particles were a structureless mess.
Some family resemblances emerged. An important distinction had to be made between particles whose spin was an
odd
integer multiple of the spin of the electron, and those whose spin was an
even
integer multiple. The reason was based on symmetry properties; the spins (in those exotic dimensions) did different things if you made the particles rotate in space. Somehow the exotic dimensions of spin and the prosaic dimensions of space were related.
The odd particles were named fermions and the even ones bosons, after two giants of particle physics, Enrico Fermi and Satyendranath Bose. For reasons that once seemed sensible, the electron spin is defined to have value ½. So bosons have integer spin (even multiples of ½ are integers) and fermions have spins ½,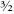 ,
,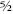 , and so on, along with their negatives â ½, â
, and so on, along with their negatives â ½, â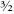 , â
, â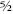 . Fermions obey the Pauli exclusion principle, which says that in any prescribed quantum system, two distinct particles cannot be in the same state at the same time. Bosons do not obey the Pauli principle.
. Fermions obey the Pauli exclusion principle, which says that in any prescribed quantum system, two distinct particles cannot be in the same state at the same time. Bosons do not obey the Pauli principle.
Fermions include all of the familiar particles: the proton, neutron, and electron are all fermions. So are more esoteric particles like the muon, tauon, lambda, sigma, xi, and omega, all names derived from the Greek alphabet. So are three types of neutrino, associated with the electron, muon, and tauon.
Bosons are more mysterious, with names like pion, kaon, and eta.
The particle physicists knew that all of these particles existed, and they could measure their physical properties. The problem was making sense of this apparent mishmash. Was the universe built from whatever happened to be to hand? Or was there a hidden plan?
The upshot of these deliberations was that many supposedly elementary particles were in fact composite. They were all made from quarks. Quarks (the name comes from
Finnegans Wake
) come in six distinct flavors, arbitrarily named: up, down, strange, charm, top, and bottom. They are all fermions, with spin ½. Each has an associated antiquark.
There are two ways to combine quarks. One is to use three ordinary quarks, in which case you end up with a fermion. The proton consists of two up quarks plus one down quark. The neutron is two down and one up. A bizarre particle called the omega-minus is made from three strange quarks. The other is to use a quark and an antiquark, which yield a boson. They don't annihilate each other because they are kept apart by nuclear forces.
For the electrical charges to work out correctly, the charges on quarks cannot be integers. Some have charge â
, some â
. Quarks come in three distinct “colors.” That makes 18 types of quark, plus 18 antiquarks. Oh, yes, there's more. We have to add some more particles to “carry” the weak nuclear force, which holds the quarks together. The resulting theory, which has great mathematical elegance despite the proliferation of particles, is known as quantum chromodynamics.
