Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (97 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

BOOK: Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine
4.12Mb size Format: txt, pdf, ePub
Jod-Basedow effect: iodine load → ↑
synthesis
of T
4
and T
3
in autonomous tissue
• Type 2 = destructive thyroiditis
↑
release
of preformed T
4
& T
3
→ hyperthyroidism → hypothyroidism → recovery
• Doppler U/S: type 1 w/ ↑ thyroid blood flow; type 2 w/ ↓ flow
• Treatment: not absolutely necessary to d/c amio b/c amio ↓ T
4
→ T
3
conversion methimazole for type 1; steroids for type 2 often difficult to distinguish so Rx for both typically initiated (
JCEM
2001;86:3) consider thyroidectomy in severely ill patient
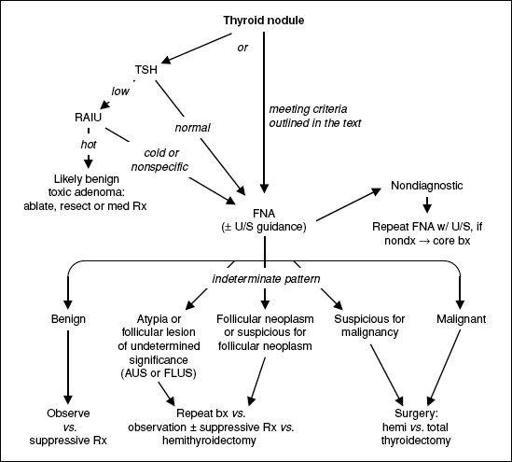
THYROID NODULES
• Prevalence 5–10% (50–60% if screen with U/S), ~5% malignant
• Features associated w/ ↑ risk of malignancy: age <20 or >70 y,, h/o neck XRT, hard and immobile mass, cold nodule on RAIU, large size, worrisome U/S findings (hypoechoic, solid, irregular borders, microcalcifications, central blood flow), cervical LAN
• Features associated w/ benign dx: FHx of autoimmune thyroid disease or goiter, presence of hypothyroidism or hyperthyroidism, nodule tenderness • Screening U/S recommended for those with FHx of MEN2 or medullary thyroid cancer, personal h/o neck XRT, palpable nodules or multinodular goiter • Any evidence of tracheal deviation or compression → ✓ PFTs & refer to surgery
• FNA for nodules >10 mm (>8 mm if irregular borders), microcalcifications or central vasculature; FNA any nodules in Pts with h/o neck XRT or FHx of MEN2 or MTC
• Indeterminate pattern in 15–30% of FNA; gene expression pattern has Se 92% & Sp 52% for malignancy (
NEJM
2012;367:705) • Suppressive Rx w/ high doses of levothyroxine less successful in iodine-sufficient regions • After complete surgical resection of thyroid cancer, RAI is administered (in Pts w/ low-risk thyroid cancer, this practice is controversial) (
Lancet
2013;381:1046 & 1058)
Figure 7-2 Approach to thyroid nodules (
Thyroid
2009;19:1167;
Am J Clin Pathol
2009;132:658)
ADRENAL DISORDERS
CUSHING’S SYNDROME (HYPERCORTISOLISM)
Definitions
• Cushing’s syndrome = cortisol excess
• Cushing’s disease = Cushing’s syndrome 2° to pituitary ACTH hypersecretion
Etiologies of hypercortisolism
• Most common is iatrogenic Cushing’s syndrome caused by exogenous glucocorticoids
•
Cushing’s disease
(60–70%): pituitary adenoma (usually microadenoma) or hyperplasia •
Adrenal tumor
(15–25%): adenoma or (rarely) carcinoma
•
Ectopic ACTH
(5–10%): SCLC, carcinoid, islet cell tumors, medullary thyroid cancer, pheo
Clinical manifestations
•
Nonspecific:
glucose intolerance or DM, HTN, obesity, oligo-or amenorrhea, osteoporosis •
More specific:
central obesity w/ extremity wasting, dorsocervical fat pads, rounded facies •
Most specific:
spontaneous bruising, proximal myopathy, wide striae, hypokalemia • Other: depression, insomnia, psychosis, impaired cognition, facial plethora, acne, hirsutism, hyperpigmentation (if ↑ ACTH), fungal skin infxns, nephrolithiasis, polyuria
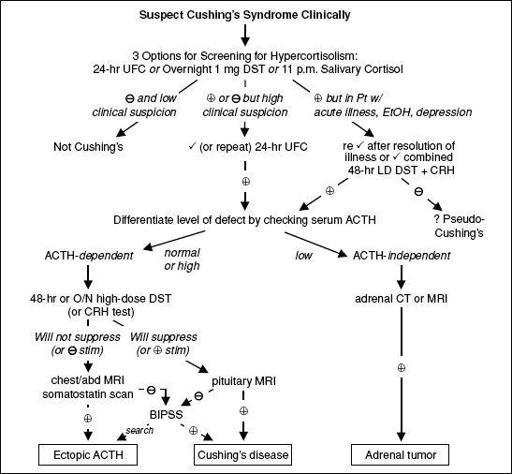
Figure 7-3 Approach to suspected Cushing’s syndrome (
nb, very difficult to diagnose as an inpatient
)
CRH, corticotropin-releasing hormone; DST, dexamethasone suppression test; UFC, urinary free cortisol Overnight 1 mg DST = give 1 mg at 11 p.m.; ✓ 8 a.m. serum cortisol (suppression if <1.8 µg/dL); 1–2% false(primarily used to evaluate subclinical Cushing’s in adrenal “incidentalomas”) (
JCEM
2008;93:1526) 11 pm salivary cortisol = abnl if level ↑; 24-h UFC = abnl if level ↑, > 4× ULN virtually diagnostic 48-h LD DST + CRH = 0.5 mg q6h × 2 d, then IV CRH 2 h later; ✓ serum cortisol 15 min later (= >1.4 µg/dL) 48-h LD DST = 0.5 mg q6h × 2 d; ✓ 24-h UFC at base. & during last 24 h of dex (suppress if <10% of base) 48-h HD DST = 2 mg q6h × 2 d; ✓ 24-h UFC as per LD DST
O/N HD DST = 8 mg at 11 p.m.; ✓ 9 a.m. serum cortisol (suppression if <32% of baseline) CRH test = 1 µg/kg IV; ✓ cortisol and ACTH (stim if > 35% ↑ in ACTH or >20% ↑ in cortisol above baseline) BIPSS, bilat. inferior petrosal sinus vein sampling; ✓ petrosal:peripheral ACTH ratio (
= 2 basal, >3 after CRH) (J Clin Endocrinol Metab 2008;93:1526)
Treatment of Cushing’s syndrome
• Surgical resection of pituitary adenoma, adrenal tumor or ectopic ACTH-secreting tumor • If transsphenoidal surgery (TSS) not successful → pituitary XRT, medical adrenalectomy w/ mitotane, or bilat surgical adrenalectomy; ketoconazole (± metyrapone) to ↓ cortisol • Glucocorticoid replacement therapy × 6–36 mo after TSS (lifelong glucocorticoid + mineralocorticoid replacement if medical or surgical adrenalectomy)
HYPERALDOSTERONISM
Etiologies
•
Primary
(adrenal disorders, renin independent increase in aldosterone) adrenal hyperplasia (60%), adenoma (
Conn’s syndrome
, 35%), carcinoma (5%) glucocorticoid-remediable aldosteronism (GRA; ACTH-dep. rearranged promoter)
•
Secondary
(extra-adrenal disorders, ↑ aldosterone is renin dependent)
Primary reninism: renin-secreting tumor (rare)
Secondary reninism
renovascular disease: RAS, malignant hypertension
edematous states w/ ↓ effective arterial volume: CHF, cirrhosis, nephrotic syndrome
hypovolemia, diuretics, T2D, Bartter’s (defective Na/K/2Cl transporterreceiving loop diuretic), Gitelman’s (defective renal Na/Cl transporter
receiving thiazide diuretic)
Other books
STRINGS of COLOR by Marian L. Thomas
The Mile High Club by Rachel Kramer Bussel
The Enigma Score by Sheri S. Tepper
La Logia de Cádiz by Jorge Fernández Díaz
Summer Season by Julia Williams
The Kingdom of Light by Giulio Leoni
Kalona’s Fall by P. C. Cast and Kristin Cast
Into the Fire (Bridge Book 2) by Meredith Wild
The Biomass Revolution (The Tisaian Chronicles) by Smith, Nicholas
Meow If It's Murder (Nick and Nora Mysteries) by T.C. LoTempio