Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (75 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

BOOK: Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine
2.7Mb size Format: txt, pdf, ePub
Treatment
• Hyperviscosity:
plasmapheresis
• Symptoms (eg, progressive anemia): rituximab ± chemotherapy (eg, cyclophosphamide, chlorambucil, fludarabine, cladribine, bendamustine) or bortezomib • Thalidomide, alemtuzumab, everolimus, ibrutinib & auto-HSCT are investigational Rx
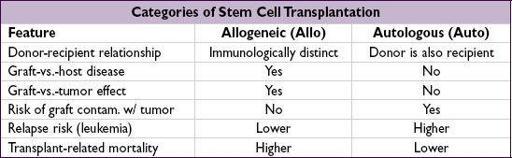
HEMATOPOIETIC STEM CELL TRANSPLANTATION (HSCT)
Transplantation of donor pluripotent cells that can reconstitute all recipient blood lineages
•
Types of Allo HSCT
:
based on donor/recipient matching of major HLA antigens on Chr. 6
(4 principal genes for serotyping:
HLA-A
,
-B
, -
C
, &
-DR
; each w/ 2 alleles ∴ 8 major Ag)
Matched related
(sibling matched at 8/8 major Ag): lowest risk of GVHD; preferred donor
Mismatched related
(eg, 1/8 Ag mismatch) or
haploidentical
(mismatch at 4/8 Ag): easiest to find, but ↑ risk of GVHD, rejection; ∴ need additional immunosuppression
Matched unrelated
: ↑ risk of GVHD; ∴ matching of 10 HLA alleles (
DQ
also
)
to ↓ risk; chance of match correlates w/ ethnicity
Umbilical cord blood
: HSC processed at birth & stored; ↓ risk of GVHD; tolerate mismatch but much slower immune reconstitution (
Blood
2010;116:4693)
•
Graft-vs.-host disease (GVHD)
:
undesirable
side effect of allo HSCT allogeneic T cells view host cells as foreign; ↑ incid. w/ mismatch or unrelated donors •
Graft-vs.-tumor (GVT)
effect:
desired
in allo-SCT; graft T cells attack host tumor cells
Indications
(
NEJM
2006;354:1813;
BMT
2010;45:1259)
•
Malignant disease
:
Auto HSCT
allows
higher ablative chemo doses
and then rescues the hematopoietic system (used mostly for lymphoma, multiple myeloma, testicular cancer)
Allo HSCT
produces
graft-versus-tumor
(GVT) effect, in addition to hematopoietic rescue (used for AML, ALL, CML, CLL, MDS, lymphoma)
•
Nonmalignant disease
: allo HSCT replaces abnl lymphohematopoietic system w/ one from nl donor (eg, immunodef., aplastic anemia, hemoglobinopathies, ? autoimmune dis.)
Transplantation procedure
•
Preparative regimen
:
chemotherapy
and/or
immunosuppression
prior to transplantation
myeloablative (traditional): chemotherapy and/or total body irradiation. Goal is
eradication
of underlying disease for which transplant is being performed.
reduced intensity conditioning (RIC or “mini”): lower dose conditioning → ↓ toxicity to allow Pts w/ comorbidities or ↑ age to tolerate HSCT. Goal to proceed w/ transplant when in disease remission. Depends mostly on GVT; ↓ mortality w/ RIC, but ↑ relapse.
•
Sources of stem cells
:
bone marrow (BM)
: original source of HSCT, now less commonly used than PBSC
peripheral blood stem cells (PBSC)
: easier collection, most commonly used source
BM vs. PBSCsurvival; BM ↓ chronic GVHD, PBSC ↓ graft failure (
NEJM
2012;367:1487)
umbilical cord blood (UCB)
: less stringent HLA-matching requirements, but fewer cells available from single donor (∴ 2 donors combined in adults); slower engraftment
haploidentical
: most available; newer regimens starting to make safer/more common
•
Engraftment
: absolute neutrophil count (ANC) recovers to 500/µL w/in
2 wk w/ PBSC,
3 wk w/ BM, ~4 wk w/ UCB. G-CSF accelerates recovery by 3–5 d in all scenarios.
Engraftment syndrome
: fever, rash, noncardiogenic pulm edema, abnl LFTs, AKI, wt gain. Dx of exclusion: r/o infection, GVHD; Rx w/ IV steroids.
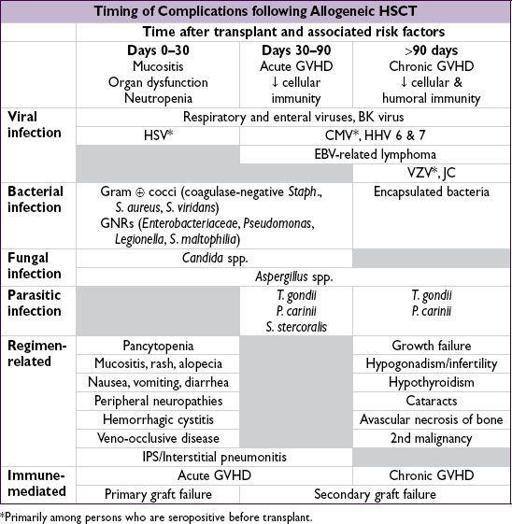
Complications
• Either
direct chemoradiotoxicities
associated with preparative regimen or consequences of
interaction between donor and recipient immune systems
•
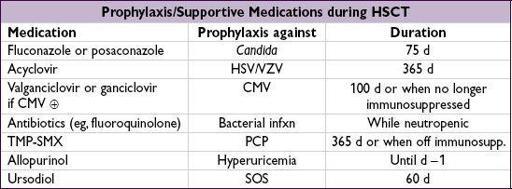
Sinusoidal obstruction syndrome (SOS):
incidence ~10%, mortality ~30%
Previously known as
veno-occlusive disease (VOD)
Mechanism: direct cytotoxic injury to hepatic venules →
in situ
thrombosis
Symptoms: tender hepatomegaly, ascites, jaundice, fluid retention with severe disease → liver failure, encephalopathy, hepatorenal syndrome
Diagnosis: ↑ ALT/AST, ↑ bilirubin; ↑ PT with severe disease; Doppler U/S
may
show reversal of portal vein flow; ↑ hepatic wedge pressure; abnl liver bx
Treatment: supportive; prophylaxis with
ursodiol
; defibrotide
•
Idiopathic pneumonia syndrome (IPS)
: up to 70% mortality (
Curr Opin Oncol
2008;20:227)
Mech: alveolar injury due to direct toxicity → fever, hypoxia, diffuse pulmonary infiltrates
Diffuse alveolar hemorrhage (DAH)
: subset of IPS
Diagnosis: bronchoscopy to exclude infection; ↑ bloody lavage fluid seen with DAH
Treatment: high-dose corticosteroids, etanercept (
Blood
2008;112:3073)
•
Acute GVHD
(usually within 6 mo of transplant;
Lancet
2009;373:1550)
Clinical grades I–IV based on scores for
skin
(severity of maculopapular rash),
liver
(bilirubin level) and
GI
(volume of diarrhea); bx supports diagnosis
Prevention:
immunosuppression
(MTX + CsA or tacrolimus) or T-cell depletion of graft
Treatment: grade I → none; grades II–IV → associated with ↓ survival and ∴ treated with immunosuppressants (corticosteroids, CsA, tacrolimus, rapamycin, MMF)
•
Chronic GVHD
(developing or persisting beyond 3 mo posttransplant)
Clinical: malar rash, sicca syndrome, arthritis, obliterative bronchiolitis, bile duct degeneration, cholestasis and many others. More common w/ PBSC than BM.
Treatment: immunosuppressants; rituximab; photopheresis
•
Graft failure
Primary = persistent neutropenia without evidence of engraftment
Secondary = delayed pancytopenia after initial engraftment; either immune mediated via immunocompetent host cells (
graft rejection
) or non–immune mediated (eg, CMV)
•
Infectious complications
due to regimen-induced pancytopenia and immunosuppression
auto HSCT recipients: no immunosuppression ∴ at ↑ risk only pre-/postengraftment
both primary infections and reactivation events occur (eg, CMV, HSV, VZV)
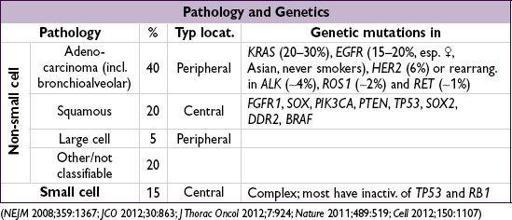
LUNG CANCER
(
NEJM
2008;359:1367;
JCO
2012;30:863;
J Thorac Oncol
2012;7:924;
Nature
2011;489:519;
Cell
2012;150:1107)
Epidemiology and risk factors
Other books
The Bridge on the Drina by Ivo Andrić
Sorcerer of the North by John Flanagan
Demon Spelled by Gracen Miller
Atlanta Heat by Lora Leigh
Unbound by Adriane Ceallaigh
Bad Hair 8 - Day Perish By Pedicure by Nancy J. Cohen
Arousing Love, a teen novel by M.H. Strom
Seer: Reckless Desires (Norseton Wolves Book 8) by Holley Trent
King Breaker by Rowena Cory Daniells