Junk DNA: A Journey Through the Dark Matter of the Genome (3 page)
Read Junk DNA: A Journey Through the Dark Matter of the Genome Online
Authors: Nessa Carey

Because of the Human Genome Project, we know where all the genes are positioned relative to one another, and their sequences. This, together with enormous improvements in the technologies used to sequence DNA, has made it much faster and cheaper to find the mutations underlying even very rare genetic diseases.
But the completion of the human genome sequence has had impact far beyond identifying the mutations that cause disease. It’s changing many of our ideas about some of the most fundamental ideas that have held sway in biology since we first understood that DNA was our genetic material.
When considering how our cells work, almost every scientist over the last six decades has been focused on the impacts of proteins. But from the moment the human genome was sequenced, scientists have had to face a rather puzzling dilemma. If proteins are so all-important, why is only 2 per cent of our DNA devoted to coding for amino acids, the building blocks of proteins? What on earth is the other 98 per cent doing?
2. When Dark Matter Turns Very Dark Indeed
The astonishing percentage of the genome that didn’t code for proteins was a shock. But it was the scale of the phenomenon that was surprising, not the phenomenon itself. Scientists had known for many years that there were stretches of DNA that didn’t code for proteins. In fact, this was one of the first big surprises after the structure of DNA itself was revealed. But hardly anyone anticipated how important these regions would prove to be, nor that they would provide the explanation for certain genetic diseases.
At this point it’s worth looking in a little more detail at the building blocks of our genome. DNA is an alphabet, and a very simple one at that. It is formed of just four letters – A, C, G and T. These are also known as bases. But because our cells contain so much DNA, this simple alphabet carries an incredible amount of information. Humans inherit 3 billion of the bases that make up our genetic code from our mother, and a similar set from our father. Imagine DNA as a ladder, with each base representing a rung, and each rung being 25cm from the next. The ladder would stretch 75 million kilometres, roughly from earth to Mars (depending on the relative positions of their orbits on the day the ladder was put in place).
To think of it another way, the complete works of Shakespeare are reported to contain 3,695,990 letters.
1
This means we inherit the equivalent of just over 811 books the length of the Bard’s
canon from mum and the same number from dad. That’s a lot of information.
If we extend our alphabet analogy a bit further, the DNA alphabet encodes words of just three letters each. Each three-letter word acts as the placeholder for a specific amino acid, the building blocks of proteins. A gene can be thought of as a sentence of three-letter words, which acts as the code for a sequence of amino acids forming a protein. This is summarised in Figure 2.1.
Each cell usually contains two copies of any given gene. One was inherited from the mother and one from the father. But although there are only two copies of each gene in a cell, that same cell can create thousands and thousands of the protein molecules encoded by a specific gene.
This is because there are two amplification mechanisms built into gene expression. The sequence of bases in the DNA doesn’t act as the direct template for the protein. Instead, the cell makes copies of the gene. These copies are very similar to the DNA gene itself, but not identical. They have a slightly different chemical composition and are known as RNA (ribonucleic acid, instead of the deoxyribonucleic acid in DNA). Another difference is that in RNA, the base T is replaced by the base U. DNA is formed of two strands joined together via pairs of bases. We could visualise this as looking a little like a railway track. The two rails are held
together by a base on one rail linking to a base on the other, as if the bases were holding hands. They only link up in a set pattern. T holds hands with A, C holds hands with G. Because of this arrangement, we tend to refer to DNA in terms of base pairs. RNA is a single-stranded molecule, just one rail. The key differences between DNA and RNA are shown in Figure 2.2. A cell can make thousands of RNA copies of a DNA gene really quickly, and this is the first amplification step in gene expression.
Figure 2.1
The relationship between a gene and a protein. Each three-letter sequence in the gene codes for one building block in the protein.
The RNA copies of a gene are transported away from the DNA to a different part of the cell, called the cytoplasm. In this distinct region of the cell, the RNA molecules act as the placeholders for the amino acids that form a protein. Each RNA molecule can act as a template multiple times, and this introduces the second amplification step in gene expression. This is shown diagrammatically in Figure 2.3.
Figure 2.2
The upper panel represents DNA, which is double-stranded. The bases – A, C, G and T – hold the two strands together by pairing up. A always pairs with T, and C always pairs with G. The lower panel represents RNA, which is single-stranded. The backbone of the strand has a slightly different composition from DNA, as indicated by the different shading. In RNA, the base T is replaced by the base U.
We can visualise this using the analogy of the knitting pattern from Chapter 1. The DNA gene is the original knitting pattern. This pattern can be photocopied multiple times, akin to producing
the RNA. The copies can be sent to lots of people who can each knit the same pattern multiple times, just like creating the protein. It’s a simple but efficient operating model and it works – one original pattern resulted in lots of soldiers with warm feet in the Second World War.

Figure 2.3
A single copy of a DNA gene in the nucleus is used as the template to create multiple copies of a messenger RNA molecule. These multiple RNA molecules are exported out of the nucleus. Each can then act as the instructions for production of a protein. Multiple copies of the same protein can be produced from each messenger RNA molecule. There are therefore two amplification steps in generating protein from a DNA code. For simplicity, only one copy of the gene is shown, although usually there will be two – one inherited from each parent.
The RNA molecule acts as a messenger molecule, carrying a gene sequence from the DNA to the protein assembly factory. Rather logically it is therefore known as messenger RNA.
Taking out the nonsense
So far, things might seem very straightforward but scientists discovered quite some time ago that there is a strange complication. Most genes are split up into bits that code for the amino acids in a protein and intervening bits that don’t. The bits that don’t are like
gobbledegook in the middle of a string of sensible words. These intervening bits of nonsense are known as introns.
When the cell makes RNA, it originally copies all of the DNA letters in a gene, including the bits that don’t code for amino acids. But then the cell removes all the bits that don’t code for protein, so that the final messenger RNA is a good instruction set for the final protein. This process is known as splicing, and Figure 2.4 shows diagrammatically how this happens.
As Figure 2.4 shows, a protein is encoded from modular blocks of information. This modularity gives the cell a lot of flexibility in how it processes the RNA. It can vary the modules which it joins together from a messenger RNA molecule, creating a range of final messengers that code for related but non-identical proteins. This is shown in Figure 2.5.

Figure 2.4
In step 1, DNA is copied into RNA. In step 2, the RNA is processed so that only the amino acid-coding regions, denoted by boxes containing letters, are joined together. The intervening junk regions are removed from the mature messenger RNA molecule.
The bits of gobbledegook between the parts of a gene that code for amino acids were originally considered to be nothing but
nonsense or rubbish. They were referred to as junk or garbage DNA, and pretty much dismissed as irrelevant. As mentioned earlier, from here on in, we’ll use the term ‘junk’ to denote any DNA that doesn’t code for protein.
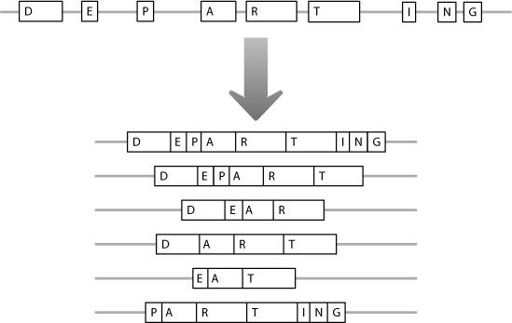
Figure 2.5
An RNA molecule can be processed in different ways. As a result, different amino acid-coding regions can be joined together. This allows different versions of a protein molecule to be produced from one original DNA gene.
But we now know that they can have a very big impact. In Friedreich’s ataxia, which we met in Chapter 1, the disorder is caused by an abnormally expanded stretch of GAA repeats in one of the junk regions, between two sections that encode amino acids. This raised the perfectly reasonable question – if the mutation doesn’t affect the amino acid sequence, why do people with this mutation develop such debilitating symptoms?
The mutation in the Friedreich’s ataxia gene occurs in the junk region between the first two amino acid-coding regions. In Figure 2.5, this would be between regions ‘D’ and ‘E’. A normal gene contains from five to 30 GAA repeats but a mutated gene
contains from 70 up to 1,000 repeated GAA motifs.
2
Researchers showed that when cells contained this expanded repeat, they stopped producing the messenger RNA encoded by the gene. Because they didn’t make messenger RNA, they couldn’t make the protein either. If you don’t send out the copies of the knitting patterns, the soldiers don’t get socks.
In fact, the cells didn’t even make the long, unprocessed RNA copy of the gene.
3
The big GAA expansion acts as a ‘sticky’ region, which prevents good copying of the DNA. It’s analogous to trying to photocopy a 50-page document, when pages four to twelve have been glued together. They won’t feed into the copier, and the process grinds to a halt, for that particular document. In the case of the Friedreich’s ataxia gene, no copying means no RNA, which means no protein.
It’s not completely clear why lack of the protein encoded by the Friedreich’s ataxia gene causes the disease symptoms. The protein seems to be involved in preventing iron overload in the parts of the cell that generate energy.
4
When a cell fails to produce the protein, the iron rises to toxic levels. Some cell types seem to be more sensitive than others to iron levels, and these include the ones affected in the disease.
A related but different mechanism accounts for Fragile X syndrome, the form of learning disability we encountered in Chapter 1. The mutation in Fragile X syndrome is the expansion of a CCG three-base repeat. Similarly to the Friedreich’s ataxia mutation, there are usually fifteen to 65 copies of the repeat on a normal chromosome. On a chromosome carrying the Fragile X mutation there are from around 200 to several thousand copies.
5
,
6
But the expansion lies in a different part of the gene in Fragile X compared with Friedreich’s ataxia. The mutation is found before the first amino acid-coding region, essentially in the junk to the left of block ‘D’ in Figure 2.5. When the junk repeat gets very large, no messenger RNA is produced,
and consequently there is no protein produced from this gene.
7
