In Search of Memory: The Emergence of a New Science of Mind (38 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology

James argued that voluntary attention is obviously a conscious process in people; therefore, it is likely to be initiated in the cerebral cortex. Viewed from a reductionist perspective, both kinds of attention recruit biological signals of salience, such as modulatory neurotransmitters, that regulate the function or configuration of a neural network.
Our molecular studies in
Aplysia
and mice support James’s contention that these two forms of attention, involuntary and voluntary, exist. One of the key differences between them is not the absence or presence of salience, but whether the signal of salience is perceived consciously. Thus I will consciously pay attention when I need to learn how to find my way from my home in Riverdale to my son Paul’s house in Westchester. But I will put my foot on the brakes automatically when a car suddenly cuts in front of me while I am driving on the road. The studies also suggest that, as James had argued, the determining factor in whether memory is implicit or explicit is the manner in which the attentional signal for salience is recruited.
In both types of memory, as we have seen, conversion of short-term to long-term memory requires the activation of genes, and in each case modulatory transmitters appear to carry an attentional signal marking the importance of a stimulus. In response to that signal, genes are turned on and proteins are produced and sent to all the synapses. Serotonin triggers protein kinase A in
Aplysia
, for example, and dopamine triggers protein kinase A in the mouse. But these signals of salience are called up in fundamentally different ways for the implicit memory underlying sensitization in
Aplysia
and the explicit memory required to form the spatial map in the mouse.
In implicit memory storage, the attentional signal is recruited involuntarily (reflexively), from the bottom up: the sensory neurons of the tail, activated by a shock, act directly on the cells that release serotonin. In spatial memory, dopamine appears to be recruited voluntarily, from the top down: the cerebral cortex activates the cells that release dopamine, and dopamine modulates activity in the hippocampus (figure 23–1).
Consistent with this idea that similar molecular mechanisms are used for top-down and bottom-up attentional processes, we have found a mechanism that may be involved in stabilizing the memory in both cases. The hippocampus of the mouse contains at least one prion-like protein similar to the one Kausik Si discovered in
Aplysia
. Martin Theis, a postdoctoral student from Germany, and I found that, in much the same way that serotonin modulates the amount and state of the CPEB protein in
Aplysia
, dopamine modulates the amount of the prion-like CPEB protein (CPEB-3) in the mouse hippocampus. This discovery raised the intriguing possibility—so far only that—that spatial maps may become fixed when an animal’s attention triggers the release of dopamine in the hippocampus and that dopamine initiates a self-perpetuating state also mediated by CPEB.
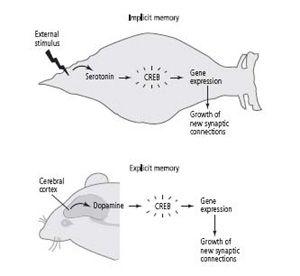
23–1 The signal of salience for long-term implicit and explicit memory.
In
implicit
(unconscious) memory, an outside stimulus automatically triggers a salience signal (serotonin) in the animal. This activates genes that lead to long-term memory storage. In
explicit
(conscious) memory, the cerebral cortex voluntarily recruits a salience signal (dopamine), which causes the animal to attend. This modulates activity in the hippocampus, leading to long-term memory storage.
THE IMPORTANCE OF ATTENTION IN STABILIZING THE SPATIAL MAP
raises another question: Is the spatial map, a map formed by learning, similar in all of us? Specifically, do men and women use the same strategies to find their way around an environment? This is a fascinating question and one that biologists are just beginning to explore.
O’Keefe, who first discovered place cells in the hippocampus, has extended his research on spatial orientation to gender differences. He has found clear differences in the way women and men attend to and orient themselves to the space around them. Women use nearby cues or landmarks. Thus when asked for directions, a woman is likely to say, “Turn right at the Walgreen’s drugstore, and then drive until you see a white colonial house on the left with green window shutters.” Men rely more on an internalized geometric map. They are likely to say, “Drive five miles north, then turn right and head east for another half mile.” Brain imaging shows activation of different areas in men and women as they think about space: the left hippocampus in men and the right parietal and right prefrontal cortex in women. These studies point out the potential benefits for group effectiveness of optimizing both strategies.
Gender differences in forming the spatial map take on additional significance when considered in a broader context: To what degree do men’s and women’s brain structures and cognitive styles differ? Are those differences innate, or do they stem from learning and socialization? It is in questions such as these that biology and neuroscience can provide us basic guidance for far-reaching social decisions.
There are many aspects of humanity that we still need to understand for which there are no useful models. Perhaps we should pretend that morality is known only to the gods and that if we treat humans as model organisms for the gods, then in studying ourselves we may come to understand the gods as well.
—Sydney Brenner, Nobel Lecture (2002)
A
nyone working on memory becomes keenly aware of the crying need for drugs that can improve memories ravaged by disease or weakened by age. But before new drugs can be brought to market, they must be tested in animal models. Clearly, given the animal models of implicit and explicit memory storage we were developing, we could begin thinking about new therapeutic approaches to memory disorders. Once again, timing proved crucial. Just when genetically modified mice were being generated in the early 1990s to analyze the nature of memory and its disorders, a new industry emerged to look for novel ways to develop drugs.
Until 1976, new scientific insights could not be translated rapidly into better modes of treatment, nor were academic scientists in the United States like myself particularly interested in working with the pharmaceutical industry to develop new drugs. In that year, however, the situation changed dramatically. Robert Swanson, a twenty-eight-year-old venture capitalist who recognized the potential of genetic engineering for developing new drugs, persuaded Herbert Boyer, a professor at the University of California, San Francisco and a pioneer in the field, to join forces with him in the formation of Genentech (short for genetic engineering technologies). This was the first biotechnology company focused on commercializing genetically engineered proteins for medical purposes. Swanson and Boyer shook hands, and each contributed $500 to the venture. Swanson then raised a few hundred thousand more to get the company off the ground. It is now worth about $20 billion.
Molecular biologists had recently discovered how to sequence DNA rapidly and had developed the powerful techniques of genetic engineering: snipping specific sequences of DNA out of chromosomes, stitching the sequences together, and inserting the recombined DNA into the genome of the
E. coli
bacterium, which makes many copies of the new gene and expresses the protein that its gene encodes. Boyer was one of the first molecular biologists to appreciate that one could use bacteria to express genes that come from higher animals, even from people. Indeed, he had been instrumental in developing some of the key techniques for doing so.
Genentech planned to use recombinant DNA technology to synthesize large quantities of two human hormones of great medical importance—insulin and growth hormone. Insulin is released into the bloodstream by the pancreas to regulate sugar in the body. Growth hormone is released by the pituitary gland to regulate development and growth. To prove that it could synthesize these two fairly complex proteins, the company first focused on a simpler protein called somatostatin, a hormone released into the bloodstream by the pancreas to turn off the release of insulin.
Before 1976 the supply of medically available somatostatin, insulin, and growth hormone was limited. Insulin and somatostatin were in short supply because they had to be purified from pigs or cows. Because the amino acid sequences of the animal hormones are slightly different from those of the human hormone, they occasionally caused allergic reactions in people. Growth hormone was derived from human pituitary glands that had been removed from cadavers. In addition to being limited, this source was occasionally contaminated by prions, the infectious proteins that cause Creutzfeldt-Jacob disease, the terrible dementia that struck Irving Kupfermann. Recombinant DNA opened up the possibility of synthesizing proteins from human genes and producing them more cheaply and in unlimited quantities without having to worry about issues of safety. It was clear to Boyer and Swanson that by cloning human genes, they could manufacture these and other medically important proteins and eventually cure genetically based illnesses by substituting cloned genes for patients’ defective ones.
In 1977, a year after joining Swanson, Boyer developed gene-cloning methods that allowed him to synthesize large amounts of somatostatin, thereby establishing the principle that recombinant DNA could produce medically important and commercially valuable drugs. Three years later, Genentech succeeded in cloning insulin.
Genentech was followed two years later by Biogen, a second powerhouse in biotechnology. But those two years had made a gigantic difference. Biogen was created not by a young entrepreneur acting initially on his own, but by C. Kevin Landry and Daniel Adams, two mature investors, each representing well-established venture groups. They brought to the table not $1000 and a handshake, but $750,000 and a set of contracts to build a biotechnology dream team. They approached the best and the brightest scientists in the world: first Walter Gilbert at Harvard, then Philip Sharp at MIT, Charles Weissman at the University of Zurich, Peter Hans Hofschneider at the Max Planck Institute for Biochemistry in Munich, and Kenneth Murray of the University of Edinburgh. After some discussion, all agreed to join the venture, and Gilbert agreed to chair the scientific advisory board.
Soon, an entire industry was launched. The biotechnology industry not only produced its own new products, it also transformed the pharmaceutical industry. In 1976 most of the big pharmaceutical companies were neither sufficiently bold nor sufficiently agile to carry off recombinant DNA research by themselves, but by investing in some biotechnology companies and buying others, they rapidly became competent.
BIOTECHNOLOGY COMPANIES ALSO TRANSFORMED THE ACADEMIC
community, particularly its attitude toward the commercialization of science. Unlike academics in most European countries, American academics had a negative atttitude toward participating in industry. France’s great biologist, Louis Pasteur, who in the nineteenth century laid the foundation for understanding that germs cause infectious disease, had many ties to industry. He discovered the biological basis for the fermenting of wine and beer. His methods of identifying and destroying bacteria that infect silkworms, wine, and milk saved both the silk and the wine industry and led to the pasteurization of milk to prevent infection and spoilage. He developed the first vaccine against rabies, and to this day the Pasteur Institute in Paris, established in his honor while he was still alive, receives a substantial part of its income from the making of vaccines. Henry Dale, the English scientist who helped discover the chemical basis of synaptic transmission, moved freely between his academic position at Cambridge University to the Wellcome Physiological Research Laboratories, a pharmaceutical company, and then back again to an academic position at the National Institute for Medical Research in London.
In America things were different. Gilbert soon realized that three conditions would induce him and other academic biologists to change their minds about combining science and business. First, they needed evidence that a company could do something useful. Second, they needed assurance that involvement in the company would not be too great a distraction from basic scientific work. Finally, they needed to be certain that their scientific independence—so valued by university professors—could not be compromised.
By 1980, when Genentech successfully produced human insulin, the first condition—that of usefulness—had been met. A steady trickle of biologists established contact with the biotechnology industry. Once these biologists had experienced sin, they found, to their surprise, that they liked it. They liked the fact that science led to medically useful drugs, and they liked the idea that they could do well financially by doing good for the public—that they could make money by developing much-needed drugs. Whereas most academics had shunned involvement with industry and disdained colleagues who consulted for pharmaceutical companies, all that changed after 1980. Moreover, academics found that given appropriate safeguards, they could limit their commitment of time and maintain their independence. Indeed, most academics found that not only did they contribute their own knowledge, they also learned new ways of doing science by working in industry.
As a result, universities started to encourage entrepreneurial skills in their faculty. Columbia was a pioneer in this regard. In 1982 Richard Axel, together with several colleagues, developed a method of expressing any gene, including a human gene, in a cell in tissue culture. Since Axel is on the Columbia faculty, the university patented the method. It was immediately adopted by several major pharmaceutical companies, which used it to make new, therapeutically important drugs. Over the next twenty years—the lifetime of the patent—Columbia earned $500 million from this one patent alone. The funds allowed the university to recruit new faculty and strengthen its research efforts. Axel and the other inventors shared in the bounty.
At nearly the same time, Cesare Milstein at the Medical Research Council Laboratory in Cambridge, England, discovered how to make monoclonal antibodies, highly specific antibodies that target just one region of a protein. His technique, too, was immediately snapped up by the pharmaceutical industry and used to make new drugs. But the Medical Research Council and Cambridge University were still thinking in an earlier mode. They did not take out a patent on the method and lost the opportunity to receive an income that they had rightly earned and that could have supported much good science. As other universities watched these events, most that did not already have an intellectual property group began to form one.
BEFORE LONG, MOST SELF-RESPECTING MOLECULAR BIOLOGISTS
had been recruited to the advisory board of one new biotechnology company or another. In this early period, companies focused mostly on hormones and antiviral agents, but by the mid-1980s, financial entrepreneurs began to wonder whether neural science could be used to produce new drugs for neurological and psychiatric disorders. In 1985, Richard Axel asked me to talk about Alzheimer’s disease at a meeting in New York City of the board of directors of Biotechnology General, a company based in Israel for which Axel consulted. I gave them a brief overview of the disorder, emphasizing that Alzheimer’s is emerging as a major epidemic because of the dramatic increase in the population over age sixty-five. Finding a treatment would have great public health benefits.
The facts I was relaying were fairly obvious to the neural science community, but they were not obvious to the venture capital community. After that meeting, Fred Adler, the chairman of the board of Biotechnology General, asked Richard and me to join him for lunch the next day. There, he proposed that we start a new biotechnology company focused exclusively on the brain, one that would use the insights of molecular science to focus on diseases of the nervous system.
At first, I was reluctant to become involved in biotechnology because I thought such an endeavor would be uninteresting. I shared the view held earlier by a large part of the academic community that biotechnology and pharmaceutical companies did humdrum science and that getting involved with a commercial venture would be intellectually unsatisfying. Richard encouraged me to join, however, pointing out that such work could be quite interesting. In 1987 we formed Neurogenetics, later called Synaptic Pharmaceuticals. Richard and Adler asked me to chair the scientific advisory board.
I asked Walter Gilbert to join the board. Wally, whom I had first met in 1984, is an extraordinary person, one of the most intelligent, gifted, and versatile biologists of the second half of the twentieth century. He had followed up on the Monod-Jacob theory of gene regulation and actually isolated the first gene regulator, showing that it was, as predicted, a protein that bound to DNA. With this remarkable accomplishment behind him, Wally went on to develop a method for sequencing DNA, which won him the Nobel Prize in Chemistry in 1980. As a cofounder of Biogen, Wally had also become knowledgeable about running a business. I thought this combination of scientific achievement and commercial know-how would make him a great asset.
Wally had left Biogen in 1984, gone back to Harvard, and turned his attention to neurobiology, a field in which he had recently become interested. Since he was new to the brain, I thought he might enjoy joining us and learning a bit more about the field. He agreed, and was an extremely valuable addition. Denise and I developed a habit that continues to this day—dining with Wally, usually at a wonderful restaurant, the night before scientific advisory board meetings.
Other scientists whom Richard and I invited to join the advisory board included Tom Jessell, our colleague at Columbia and a gifted developmental neurobiologist; Paul Greengard, a pioneer in second-messenger signaling in the brain who had moved from Yale to Rockefeller University; Lewis Roland, chairman of the neurology department at Columbia; and Paul Marks, former dean of the College of Physicians and Surgeons at Columbia and subsequently president of Memorial Sloan-Kettering Cancer Center. This was an extraordinarily strong group. We spent several months studying what direction the company should take.
We first considered specializing in amyotrophic lateral sclerosis, of which Alden Spencer had died, and then in multiple sclerosis, brain tumors, or stroke, but we eventually decided it would probably be best to do something related to receptors for the neurotransmitter serotonin. Many important drugs—almost all of the antidepressants, for example—act through serotonin, and Richard had just isolated and cloned the first serotonin receptor. Unlocking the molecular biology of these receptors could open up the study of a number of diseases. Moreover, the receptor cloned by Richard was only one of a large class of metabotropic receptors, so it could be used to try to clone similarly constructed receptors for other transmitters that act through second messengers.
We were strongly encouraged to work along these lines by Kathleen Mullinex, the associate provost at Columbia, whom we had recruited as chief executive officer. Although Mullinex knew no neurobiology, she had the idea that receptors could be useful to screen for new drugs. The board sharpened that idea. We would clone receptors for serotonin and dopamine, see how they function, and then design new chemical compounds to control them. Paul Greengard and I wrote the document that spelled this out, and we used as our first example Richard Axel’s successful cloning of the first serotonin receptor.
