In Search of Memory: The Emergence of a New Science of Mind (39 page)
Read In Search of Memory: The Emergence of a New Science of Mind Online
Authors: Eric R. Kandel
Tags: #Psychology, #Cognitive Psychology & Cognition, #Cognitive Psychology

The company got off to a good start. We recruited a fine scientific staff, which proved adept at cloning new receptors, and we formed effective partnerships with Eli Lilly and Merck. The company went public in 1992 and disbanded its extraordinary scientific advisory board. I remained for a while as a scientific consultant, but three years later I started a company focused on my own area of research.
THE IDEA FOR THIS NEW VENTURE ORIGINATED ONE NIGHT IN
1995, as Denise and I were having one of our dinners with Walter Gilbert. Wally and I were discussing results I had recently obtained suggesting that memory loss in aged mice can be reversed, when Denise suggested that we start a company to develop a “little red pill” for age-related memory loss. Following up on this idea, Wally and I joined forces with Jonathan Fleming, a venture capitalist from the Oxford Partners group who had supported Synaptic Pharmaceuticals. Jonathan helped us recruit Axel Unterbeck from Bayer Pharmaceuticals. In 1996 the four of us formed a new company, Memory Pharmaceuticals.
Starting a company based so directly on my work in memory was exciting, but running a company, even one derived from one’s own research, is exceedingly time-consuming. Some academics leave the university in order to do it. I had no intention of leaving Columbia or the Howard Hughes Medical Institute. I wanted to help found the company and, once that was done, consult for it on a part-time basis. Both Columbia and Howard Hughes have experienced lawyers who helped me work out consulting agreements—first with Synaptic Pharmaceuticals and then with Memory Pharmaceuticals—that met both institutional guidelines and my own concerns.
Participating in these two biotechnology companies expanded my horizons. Memory Pharmaceuticals allowed me to help translate my basic research into potentially useful drugs for treating people. In addition, it exposed me to how a company works. In a typical academic department, junior faculty members are independent; in the early stage of their career they are encouraged not to collaborate with senior faculty but to develop their own research programs. In business, people must work together for the good of the company using intellectual and financial resources in a way that pushes each potential product in promising directions. Although this cooperative characteristic of industry is generally not found in universities, there are important exceptions, such as the Human Genome Project, which involved a similar merging of individual efforts for a common good.
The new company was based on the idea that the study of memory will expand into an applied science and that one day our growing understanding of the mechanisms of memory function will lead to treatments for disorders of cognition. As I had pointed out to the board of Biotechnology General, disorders of memory are more evident today than they were when I began practicing medicine fifty years ago because people are living longer now. Even in a normal, healthy population of seventy-year-olds, only about 40 percent have as good a memory as they had in their mid-thirties. The remaining 60 percent experience a modest decline in memory. In the early stages, this decline does not affect other aspects of cognitive function—it does not affect language or the ability to solve most problems, for instance. Half of the 60 percent have a slight memory impairment, sometimes called benign senescent forgetfulness, that progresses only slowly, if at all, with time and age. The remaining half, however (or 30 percent of the population over age seventy), develop Alzheimer’s disease, a progressive degeneration of the brain.
In its early stages, Alzheimer’s is characterized by mild cognitive impairment that is indistinguishable from benign senescent forgetfulness. But in later stages of the disease, dramatic and progressive deficits in memory and other cognitive functions develop. The vast majority of symptoms in the late, debilitating stages of the disease are attributed to the loss of synaptic connections and to the death of nerve cells. This degeneration of tissue is caused in large part by the accumulation of an abnormal material known as β-amyloid in the form of insoluble plaques in the spaces between brain cells.
I FIRST TURNED MY ATTENTION TO BENIGN SENESCENT FORGETFULNESS
in 1993. The term is a bit euphemistic, since the disorder does not begin with senescence nor is it completely benign. It first becomes evident in some people in their forties and typically becomes slightly more pronounced with time. I hoped that the ever expanding understanding of the mechanisms of memory storage in
Aplysia
and mice might enable us to understand the underlying defect of this distressing aspect of aging and then to develop therapies for counteracting the memory loss.
As I read the literature on benign senescent forgetfulness, it became clear to me that the disorder is similar in character, if not in severity, to a memory deficit associated with damage to the hippocampus: the inability to form new long-term memories. Like H.M., people with benign senescent forgetfulness can carry on a normal conversation and retain ideas in short-term memory, but they cannot readily convert new short-term memory into long-term memory. For example, an elderly person who is introduced to someone new at a dinner party may remember the new name for a short while, but forget it completely by the next morning. This similarity gave me the first clue that age-related memory loss may involve the hippocampus. Later examination of people and of experimental animals revealed that this is in fact the case. An additional clue was provided by the finding that there is, with aging, a loss of synapses that release dopamine in the hippocampus. We had earlier found that dopamine is important for maintaining long-term facilitation and for modulating attention in spatial memory.
To obtain a better understanding of this form of memory loss, my colleagues and I developed a naturally occurring model of it in the mouse. Laboratory mice live to be two years old. Thus, mice are young when they are three to six months old. At twelve months, they are middle-aged, and at eighteen months, elderly. We used a land maze similar to the one we had used earlier to examine the role of genes in spatial memory. Placed in the center of a large circular platform surrounded by a rim of forty holes, mice learn to find the one hole that leads to an escape chamber by discovering the spatial relationship between the hole and markings on the wall. We found that most young mice rapidly go through random and serial escape strategies and learn to use the more efficient spatial strategy rather soon. Many aged mice, however, have difficulty ever learning the spatial strategy (figure 24–1).
We also found that not all older mice are impaired: the memory of some is as good as that of young animals. In addition, the memory deficit in impaired mice occurs just in explicit memory; we carried out a number of behavioral tests and found that their implicit memory for simple perceptual and motor skills was unaffected. Finally, the memory deficits are not necessarily confined to old age; some began in midlife. All of these findings suggested to us that what pertains to people also pertains to mice.
If a mouse has a defect in spatial memory, it implies that something is wrong with the hippocampus. We explored the Schaffer collateral pathway in the hippocampus of older mice with age-related memory deficits and found that the late phase of long-term potentiation, which we and others had found to be strongly correlated with long-term explicit memory, was defective. Moreover, older mice that remembered well had normal long-term potentiation, as did younger mice with normal spatial memory.
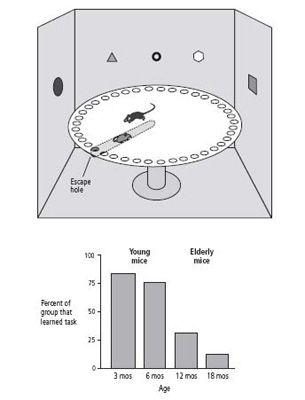
24–1 Mice show age-related memory loss in a spatial task
. The Barnes Maze (above) provides an escape hole and several visual cues to orient the mouse. Aged mice have difficulty learning the spatial relationships between these cues and the escape hole (below). This correlates with defective functioning of the hippocampus.
We had found earlier that the late phase of long-term potentiation is mediated by cyclic AMP and protein kinase A and that this signaling pathway is activated by dopamine. When dopamine binds to its receptor in the pyramidal cells of the hippocampus, the concentration of cyclic AMP increases. We found that drugs which activate these dopamine receptors, and thereby increase cyclic AMP, overcome the deficit in the late phase of long-term potentiation. They also reverse the hippocampus-dependent memory deficit.
Mark Barad, a postdoctoral fellow, and I wondered whether the deficit in older mice’s long-term spatial memory might also be ameliorated by manipulating the cyclic AMP pathway in another way. Cyclic AMP is normally broken down by an enzyme so that signaling does not continue indefinitely. The drug Rolipram inhibits that enzyme, extending the life of cyclic AMP and increasing signaling. In old mice, Barad and I found, Rolipram significantly improves learning that involves the hippocampus; indeed, older animals given Rolipram performed as well as younger mice on memory tasks. Rolipram even increased long-term potentiation and hippocampus-dependent memory in young animals.
These results support the notion that the decline in hippocampus-dependent learning in older animals is due, at least in part, to an age-related deficit in the late phase of long-term potentiation. Perhaps more important, they suggest that benign senescent forgetfulness may be reversible. If it is, the elderly may be treated in the near future with drugs developed from such studies of the mouse.
The prospect that benign senescent memory loss is treatable led the leadership of Memory Pharmaceuticals to wonder what other forms of memory impairment might be treated if we knew more about the molecular mechanisms underlying memory formation. With this idea in mind, Memory Pharmaceuticals turned its attention to the early phase of Alzheimer’s disease.
ONE OF THE INTERESTING FEATURES OF ALZHEIMER’S DISEASE
is the mild deficit in memory that precedes the deposition of β-amyloid plaques in the hippocampus. Since the early cognitive deficits in Alzheimer’s are so similar to age-related memory loss, Michael Shelanski at Columbia began to wonder whether the same pathways are disturbed in each. To find out, he studied the hippocampus of mice.
He exposed the mouse hippocampus to the most toxic component of β-amyloid plaques, known as the Aβ peptide, and found that long-term potentiation was impaired before any neurons had died or plaques had formed. In addition, animal models of early Alzheimer’s disease displayed memory deficits before any detectable accumulation of plaque or evidence of cell death. While examining gene expression in hippocampal cells exposed to the Aβ peptide, Shelanski discovered that the peptide decreases the activity of cyclic AMP and protein kinase A. This finding suggested to him that the peptide may compromise the cyclic AMP-protein kinase A system. Indeed, he found that increasing cyclic AMP via Rolipram prevents Aβ toxicity in mouse neurons.
The same drugs that prevent age-related memory loss in mice also prevent memory deficits in mice in the early stages of Alzheimer’s disease. Ottavio Arancio from Columbia University went on to show that Rolipram protects against some of the damage to neurons sustained in Alzheimer’s, thereby suggesting that cyclic AMP not only strengthens the function of pathways whose efficiency has declined, but also helps protect against nerve cell damage and perhaps even leads to regeneration of lost connections in the mouse model of Alzheimer’s disease.
Memory Pharmaceuticals and other companies developing drugs to combat memory loss are now tackling both of these disorders. Indeed, most companies have broadened their base since their founding and are now developing drugs not only for age-related memory loss and Alzheimer’s disease, but also for a variety of memory problems that accompany other neurological and psychiatric disorders. One such disorder is depression, which in its more severe forms is associated with a dramatic loss of memory. Another is schizophrenia, which is characterized by a defect in working memory and in executive functions, such as ordering a sequence of events or attending to priorities.
MEMORY PHARMACEUTICALS IS NOW LOCATED IN MONTVALE, New Jersey. In 2004 the company went public. It has developed four new families of drugs for age-related memory loss that are substantially better than the off-the-shelf compounds my colleagues and I at Columbia had used for our experiments. Some of the compounds improve a rat’s memory of a new task for months!
The era of biotechnology holds enormous promise for developing new drugs to treat people with mental diseases. In another decade we may find that our understanding of the molecular mechanisms that underlie memory formation has led to therapeutic advances that were scarcely imaginable in the 1990s. The therapeutic implications of these drugs are obvious. Less obvious are the effects the biotechnology industry will have on the new science of mind and on academic life. Not only do academics serve on advisory boards, but some of the very best scientists are leaving superb jobs at universities to take what they perceive are even better jobs in biotechnology. Richard Scheller, the extraordinary molecular biologist who was the postdoctoral fellow working with Richard Axel and with me when we began our efforts to apply molecular biology to the nervous system, left Stanford University and Howard Hughes Medical Institute to become vice president for research at Genentech. He was joined shortly thereafter by Marc Tessier-Lavigne, an outstanding developmental neurobiologist from Stanford. Corey Goodman, an acknowledged leader in the study of the development of the
Drosophila
nervous system, left the University of California, Berkeley to run his own company, Renovis. The list goes on.
