The Cerebellum: Brain for an Implicit Self (26 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

In humans, ankle stretch evokes a reflex contraction of calf (soleus and gastrocnemius) muscles. In view of its long latency (120 ms), this response must be mediated by a supraspinal pathway, which is called the “functional (or long-latency) stretch reflex.” This reflex has task and context dependencies. For example, Nashner (
1976
)
showed that when a human standing upright on a platform was subjected to a sudden backward movement of the platform, the functional stretch reflex was evoked in calf muscles, which helped reduce postural sway. On the other hand, when the platform was suddenly inclined forward and upward, the reflex was depressed because it would have enhanced a backward postural sway. Following an unexpected change in the usefulness of stretch reflexes, certain subjects progressively altered reflex gain during the succeeding three to five trials, always in the direction that optimized the reflex response. The cerebellum appeared to be involved in assessing the stabilizing effect of the functional stretch reflex because clinically diagnosed cerebellar deficits diminished the extent of reflex adaptation (
Nashner, 1976
). The functional stretch reflex might be produced in a neuronal circuit involving the B-zone, but this issue is still open.
A subtle mechanism, presumably involving the cerebellum, has been suggested to operate in humans standing freely while maintaining a stable, quiet posture. When small, unobtrusive mechanical perturbations, mimicking those occurring naturally, were applied to the foot using a piezoelectric translator, the posture remained stable but the perturbations produced no stretch reflex response in calf muscles. Also, intrinsic (non-neural) ankle stiffness of the foot, attributable to the Achilles’ tendon and several aponeuroses, was shown to be insufficient to stabilize the body. Hence, the stabilization must have been maintained by additional neural modulation of ankle torque (
Loram and Lakie, 2002
). This modulation may have been provided by predictive control using a cerebellar internal model (
Loram et al., 2005
).
Noxious stimuli applied to the skin of a cat forelimb causes flexion of that forelimb. These stimuli evoke mossy fiber and climbing fiber signals, which reach the C
1
and C
3
zones of lobule V. Purkinje cells in these areas project to the anterior interpositus nucleus, which, in turn, projects to the magnocellular red nucleus and primary motor cortex. Finally, descending signals along the rubrospinal and corticospinal tracts evoke withdrawal of the forelimb (
Figure 33
illustrates only the red nucleus pathway). The lobule V/C
3
zone contains 30–40 longitudinal microzones lying side by side, each 50–150 micrometers wide. Purkinje cells in each microzone receive simple-spike inputs from a small receptive field on the skin and adjust the withdrawal movements to be appropriate for avoiding the stimuli to this part of the skin. If not appropriate, the nociceptive stimuli may not be avoided and may stimulate climbing fiber receptive fields. Responses of Purkinje cells to such inappropriate stimuli could then be suppressed by the induction of conjunctive LTD. Climbing fibers in adjacent microzones are activated from adjacent skin areas, forming a detailed somatotopic map of the ipsilateral forelimb’s skin, particularly
its distal parts. Adjacent microzones innervate, in turn, adjacent cell groups in the anterior interpositus nucleus. Through further projections to the red nucleus, these microzones control movement components that have specific relationships with the location of climbing fiber receptive fields (
Garwicz et al., 1998
;
Ekerot and Jörntell, 2003
) (
Chapter 9
, “
Network Models
”).
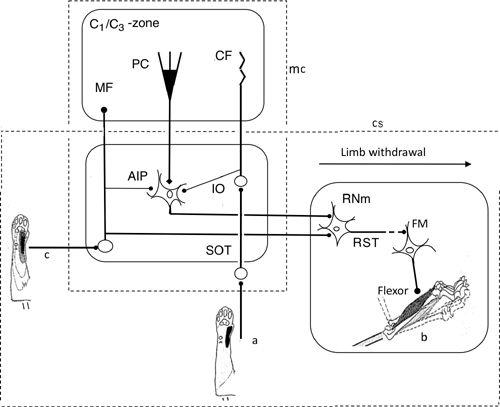
Figure 33. Neural wiring diagram for the limb withdrawal reflex.
The cerebellum provides adaptive control to optimize limb withdrawal in response to nociceptive stimulation of the skin and/or other leg parts. In this case, cerebellar nuclear neurons are in the position of the controller, while the magnocellular portion of red nucleus (RNm) is nested in the controlled object. In spite of this complexity, the general framework of the adaptive cerebellar control applied to the VOR (
Figure 28
) and stretch reflex (
Figure 32
) is applicable to the limb’s withdrawal. Abbreviations: a, CF receptive field in the forearm; AIP, anterior interpositus nucleus; b, forelimb flexor muscle; c, MF receptive field; C
1
, C
3
, names of the longitudinal cerebellar zones involved in withdrawal reflexes; CF, climbing fiber; FM, flexor motoneuron; MF, mossy fiber; PC, Purkinje cell; RST, rubrospinal tract. RST is interrupted to indicate that its connection to FM is mediated by segmental interneurons. (Based on
Apps and Garwicz, 2005
.)
In the classic half center model of a CPG, as proposed by Anders Lundberg and his colleagues (
Jankowska et al., 1967a
,
b
;
Stuart and Hultborn, 2008
), it was assumed
that each limb is controlled by a separate half center and that each half center contains two groups of excitatory interneurons that directly project to flexor and extensor motoneurons, respectively. Because of the mutual inhibitory interconnections between the two groups, only one group can be active at a time (
Figure 34
). The CPG circuit for lamprey locomotion has recently been analyzed in depth and reconstructed by simulation (
Grillner et al., 1991
,
2007
;
Grillner and Jessel, 2009
). A substantial but less complete model has also been achieved for cat locomotion (
McCrea and Ryback, 2008
).
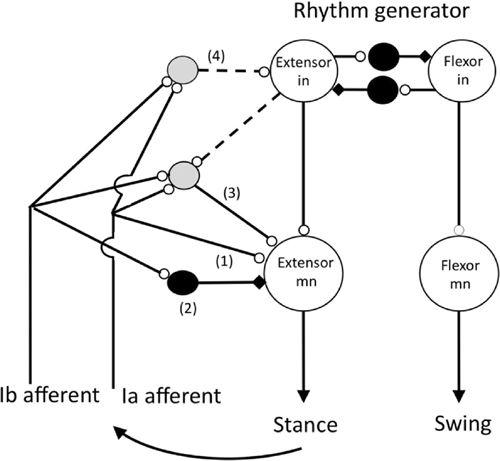
Figure 34. The organization of reflex pathways from spindle Ia afferents in extensor muscles to motoneurons supplying the same muscles during walking in the cat.
In this schematic, note the large and medium-sized hollow grey circles, and the small hollow circles that represent excitatory neurons and excitatory synapses, respectively. Likewise, the middle-sized filled circles and the small black rectangles represent inhibitory neurons and inhibitory synapses, respectively. Pathways (1) and (2) are the well-known monosynaptic excitatory and disynaptic inhibitory pathways from group 1a and 1b afferents, respectively, that were first revealed in barbiturate anesthetized in vivo cat preparations. Excitatory pathways (3) and (4) are open during locomotion and probably most other natural movements. Transmission in the disynaptic excitatory pathway (3) occurs during extension where it presumably functions, for example, to reinforce ongoing extensor activity during the stance phase of the step. One function of pathway (4) is to regulate the duration of the stance phase of the step by exciting the extensor half center of the locomotor rhythm generator. Activity in group 1b afferents during the stance phase of the step prevents the onset of the swing (flexion) phase of the step until the extensor muscles are unloaded. The shaded interneurons in pathways (3) and (4) have not yet been identified, and the connections indicated by the dashed lines are the most parsimonious for describing the functional effect of extensor group I afferents. The rhythm generator is assumed to include mutually inhibiting extensor and flexor half centers. (Based on Pearson, 1995;
Hultborn, 2001
;
Stuart and Hultborn, 2008
;
Nichols and Ross, 2009
.)
The involvement of the cerebellum in locomotion had been suggested by classic lesion studies in quadrupeds (
Chapter 2
, “
Traditional Views of the Cerebellum
”), which impaired coordination of the four limbs and other parts of the body as well. The modern approach to studying the mechanisms of locomotion started when steady quadrupedal locomotion was successfully evoked in high decerebrate cats on a treadmill belt during repetitive electrical stimulation of a specific site within the midbrain, the mesencephalic locomotor center (
Shik et al., 1966
). Stimulation of a restricted region along the midline cerebellar white matter in a decerebrate cat was later shown to evoke a generalized augmentation of postural muscle tone on a stationary treadmill belt and locomotion on a moving one. This effect appeared to be mediated by crossed fastigioreticular fibers that excited presumably the same reticulospinal pathway activated by the mesencephalic locomotor center (
Mori et al., 1999
).
During mesencephalic-locomotor-center-evoked locomotion in the high decerebrate cat, there is phasic activity in rubro- (
Orlovsky, 1972b
), reticulo- (Perreault et al., 1993), and vestibulospinal (
Orlovsky, 1972a
) pathways. This rhythmic activity is dependent on an intact cerebellum and appears to be mediated by spinocerebellar pathways conveying information on the activity of the locomotor-generating spinal networks (Arshavsky et al., 1983, Arshavsky et al., 1986). Similar results on movement-related phasic activity in rubro- and vestibulospinal neurons have also been described for fictive scratching (
Arshavsky et al., 1978a,b
,
1988
).
Spike discharges have also been found to occur in DSCT and VSCT neurons in synchrony with motor rhythms (cat; Arshavsky et al., 1972a,b) (
Chapter 6
, “
Pre- and Post-Cerebellar Cortex Neurons
”). Magnocellular red nucleus neurons, the origin of the rubrospinal tract, also exhibited spike discharges related to each cycle of locomotion. The average firing frequency was minimal at the transition between the rhythmical alternating discharge of extensor and flexor efferents, and it increased progressively to reach a maximum in the second phase of the flexor burst (
Arshavsky et al., 1988
). Apparently, the rubrospinal tract mediates the function of the C
1
/C
3
zone via the anterior interpositus nucleus when it adaptively controls the swing phase of limbs during locomotion. Lateral vestibulospinal tract neurons were
also found to discharge during controlled locomotion. Most of the neurons projecting to the cervical segments were shown to have two frequency modulation peaks in their discharge: one in the late swing or early stance phase of a step by the ipsilateral forelimb and the other in the late stance or early swing phase (
Udo et al., 1982
). In decerebrate cats, a stretch reflex of the soleus muscle and its H-reflex were reported to be modulated during quadrupedal treadmill locomotion, reaching its peak at or before the peak in soleus EMG activity associated with the stance phase of the step (
Akazawa et al., 1982
). Marked modulation of the H reflex in the soleus muscle has also been demonstrated in humans during walking and running (
Edamura et al., 1991
). Evidence indicated that the locomotion-related modulation represented postsynaptic changes in α-motoneuron activity or in presynaptic inputs to these motoneurons, but not to changes in muscle spindle discharge (
Akazawa et al., 1982
). The modulation of stretch reflexes observed during controlled locomotion might be induced, at least partly, via the lateral vestibulospinal tract whose activity is, itself, known to be modulated by locomotion (
Udo et al., 1982
).
In B-zone Purkinje cells, Udo et al. (
1981
) showed that climbing fiber responses occurred during controlled locomotion. During stable locomotion at a belt velocity of 36 centimeters per second, climbing fiber responses were modulated slightly: a small increment in their discharge rates occurred during the swing phase of the ipsilateral forelimb (
Yanagihara and Udo, 1994
). In an experiment on decerebrate ferrets, the perturbation of locomotion by a bar extended into the trajectory of the right forelimb in a specific phase of the step cycle also caused climbing fiber discharges (
Lou and Bloedel, 1992
). In mice, the extent to which their locomotion on a normal treadmill kept pace with increasing belt speed was taken as a measure of adaptation. This distinguished control mice from mutants deficient in mGluR1, which is required for conjunctive LTD induction (
Ichise et al., 2000
). In another experiment, a cat walking on a horizontally placed ladder was perturbed when one of the stepped-on rungs dropped 2 centimeters. Such drops usually resulted in some Purkinje cells generating climbing fiber responses at a short latency. Importantly, these responses were well time-locked to the onset of the unexpected rung drops but not to their cessation, which they often preceded (
Andersson and Armstrong, 1987
).
