The Cerebellum: Brain for an Implicit Self (28 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

Several areas of the cerebellum are now known to affect cardiovascular functions (
Nisimaru, 2004
), with two of them studied in detail. One is the lateral edge of lobules X (nodulus) and IX (uvula), which is linked to the vestibular nucleus and a medial part of the parabrachial nucleus. It functions in the vestibulosympathetic reflex. The other is folium-p in the flocculus (
Figure 28
), which connects with the lateral part of the parabrachial nucleus and functions in connection with the somatosympathetic reflex.
The vestibulosympathetic reflex acts to maintain blood pressure stability during head and body movement. For example, head tilting with the nose upward in awake rabbits causes a slight decrease in mean arterial blood pressure, which recovers to the control level within 3–5 seconds. The lateral nodulus-uvula is considered to adaptively improve the dynamics of the vestibulosympathetic reflex. Indeed, after bilateral lesioning of the lateral nodulus-uvula, recovery is retarded in the just-cited head-tilt-evoked blood pressure changes (
Nisimaru et al., 1998
). The most lateral zone of the nodulus and uvula receives mossy fiber afferents from the ipsilateral vestibular nuclei and climbing fiber afferents from the contralateral vagal and aortic nerves via the medial accessory olive (MAO). This zone, in turn, sends Purkinje cell axons to the parabrachial nucleus and vestibular nuclei. These connections subserve adaptive control of the vestibulosympathetic reflex.
The somatosympathetic reflex has a very different role. During movement, it helps redistribute arterial blood flow among working and non-working muscles and viscera. For example, during defense reactions of a rabbit to electric foot shocks, the rate of arterial blood flow to active muscles increases, whereas it decreases to inactive muscles and viscera. The entire neuronal circuit for this behavior has recently been analyzed in detail (
Nisimaru et al., 2010
). The reflex arises from the activity of high-threshold afferents supplying muscles, joints, and skin. This activity is conveyed to the parabrachial nucleus, the coordinating center of the reflex, whose output, in turn, activates the descending sympathetic system. Therefore, the folium-p appears to be embedded to the somatosympathetic reflex as its adaptive mechanism. Interestingly, orexin-containing beaded fibers arise from the hypothalamus and pass to the flocculus, most densely in the folium-p. Here, orexins appear to act as neuromodulator in the fight-or-flight situations of defense reactions invoving the somatosympathetic reflex and folium-p (
Nisimaru et al., 2006
,
2010
).
The involvement of the cerebellum in respiration has long been discussed. In a recent study on anesthetized rats, stimulation of the fastigial nucleus, but not the interpositus and lateral nuclei, significantly affected respiration, primarily by increasing its frequency. Lesions of cerebellar nuclei by microinjection of kainic acid did not significantly alter eupneic breathing, but fastigial nuclear lesions attenuated the respiratory responses to hypercapnia and sodium cyanide. This implies that fastigial nuclear neurons uniquely modulate respiration independent of cardiovascular effects and facilitate respiratory responses mediated by activation of CO
2
and O
2
receptors. Hence, it has been suggested that a microcomplex in the A-zone-fastigial system functions to make the respiratory chemoreflexes adaptive (Xu and Frazier, 2000).
An ongoing study has revealed prominent respiratory rhythmic activity emanating from the cerebellar cortex in newborn rats, using block preparations of the cerebellum-pons-medulla-spinal cord. These blocks were excised from rats at postnatal week 1 and maintained in a perfusion chamber. Optical imaging with voltage-sensitive dyes and whole cell patch clamp recording from Purkinje cells showed that a respiratory rhythm was present in many parts of the cerebellum, especially in the lateral part of paraflocculus and lateral end of the vermis. Similar inspiratory and postinspiratory activities were seen in the dorsal part of the IO and the parabrachial nucleus (
Arata et al., 2009
). The functional meaning of these respiratory activities, which do not occur in the adult, is unclear. It is nonetheless an interesting observation suggesting developmental changes deserving of full study.
After reading
Chapters 10
and
11
, one might think that the cerebellum originally evolved to facilitate reflexes becoming readily adaptable to ever-changing environments. Indeed, studies of reflexes do provide simple, robust experimental paradigms for evaluating the mechanisms and roles of cerebellar circuits. These paradigms will continue to be useful, particularly when testing cerebellar functions in behaving animals and patients with cerebellar lesions.
After reviewing the involvement of the cerebellum in various reflexes in
Chapters 10
and
11
, we can now address the question of how the intricate neuronal circuit integrated in the form of a microcomplex is incorporated into reflex control systems for a subtle adaptive mechanism. Here, we consider major adaptive control system models proposed to this point, and the experimental evidence on which these models are based.
In various ocular reflexes, neuronal circuit components corresponding to the controller, controlled object, and adaptive mechanism have been identified on the basis of anatomical structures, lesion effects, and activities of Purkinje cells and other involved neurons. These components are illustrated in
Figures 36
to
38
. As reviewed in
Chapter 10
, “
Ocular Reflexes
,” visual feedback plays no role in the VOR. It may operate in the OKR, OFR, and saccades, but only quite inefficiently because of its relatively long delay (50–100 milliseconds) (
Smith et al., 1969
) (see
Chapter 10
). Hence, to maintain an optimal gain over a given range of vestibular, visual, and oculomotor conditions, these ocular reflexes need the intervention of a microcomplex as an adaptive controller.
Figure 36. Control system scheme for adaptation of the vestibuloocular reflex.
The block diagram for adaptive VOR control is based on the neuronal wiring diagram in
Figure 28
. The shade on the left is for the sensory system (Ss) processing information for the controller. The shade in the middle encloses the microcomplex (Ac), which uses VN and the flocculus to act as an adaptive controller. The shade on the right is for the controlled object (Co). The dotted arrow represents climbing fibers. Abbreviations: a, sensory mossy fiber pathways; AOS, accessory optic system; b, recurrent mossy fiber pathways (shown by broken lines because of the ambiguity of their presence); IO, inferior olive; OM, oculomotor neurons; VN, VOR relay neurons in the vestibular nuclei; Ve, eye velocity; Vh, head velocity. (Based on
Ito, 1972
,
1974
,
1998
,
2006
.)
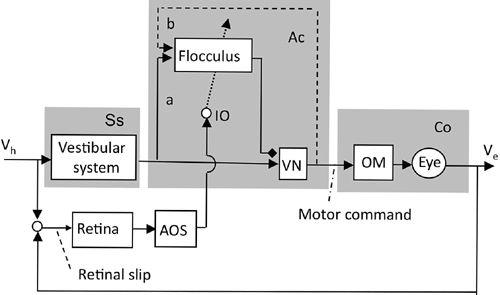
Figure 37. Control system scheme for adaptation of the optokinetic reflex.
The OKR shares the controller, controlled object, and flocculus with the VOR but responds to visual signals. This is an example of a multi-input reflex system, as explained in
Figure 9A
. Abbreviations: NRTP, nucleus reticularis tegmenti pontis; Vs, screen velocity. For other abbreviations and symbols, see
Figure 36
. (Based on
Ito, 2006
.)
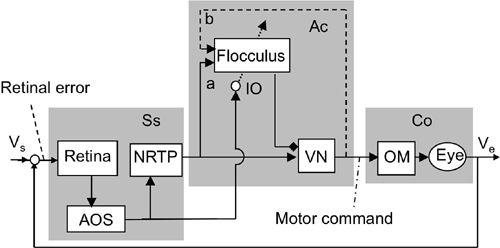
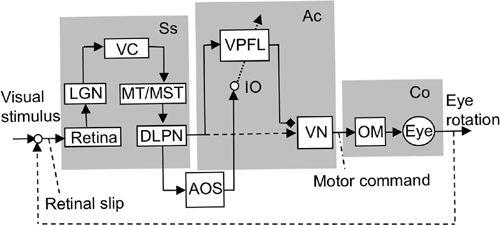
Figure 38. Control system scheme for the ocular following response.
This block diagram shows that adaptive control of the OFR involves the cerebral cortex (VC/MT/MST) and the ventral paraflocculus (VPFL). Other abbreviations: DLPN, dorsolateral pontine nucleus; LGN, lateral geniculate nucleus; MST, medial superior temporal area; MT, medial temporal area; VC, visual cortex. For further abbreviations, see
Figure 36
. The possible connection from DLPN to VN is indicated by a broken line because it has not been confirmed. (Based on
Kawato, 1999
;
Ito, 2006
.)
In both the VOR and OKR, the same group of vestibular neurons acts as the common controller (
Figures 36
and
38
), which is the target of flocculus Purkinje cells. The controller for the OFR has not been well explored, but it should consist of neurons targeted by ventral paraflocculus Purkinje cells (
Figure 38
). Because
these Purkinje cells project to vestibular nuclei in a pattern similar to that of flocculus Purkinje cells (
Balaban et al., 1981
), it is conceivable that a group of vestibular nuclear neurons serve as the controller of the OFR (
Chapter 10
). This group of vestibular neurons could be identical or, even if not, at least akin to VOR/OKR relay neurons. The combination of the VOR, OKR, and OFR provides a good example of a multi-input control system, that is, sharing the controller and the controlled object (
Figure 9A
). Note that even though the OFR involves the parietal association cortex (MT/MST area), it is an elaborate visual perception mechanism and by no means designed for voluntary motor control.
In the flocculus hypothesis first proposed in the early 1970s, we assumed that the primary drive to flocculus Purkinje cells came from sensory signals originating in the vestibular organ (
Ito, 1972
,
1982
). In this hypothesis, the flocculus is a modifiable side path to the major VOR pathway, as schematically shown in
Figure 39A
. Miles and Lisberger (
1981
) challenged this hypothesis, as schematically shown in
Figure 39B
, and the debate that followed centered on two issues (
Ito, 1993a
;
Lisberger and Sejnowski, 1992
,
1993
). The first concerned the source and functional role of eye velocity signals in flocculus Purkinje cells. The second issue (discussed in the next section) concerned the memory sites for VOR adaptation. The first issue required asking whether eye velocity signals were mediated by recurrent collaterals of VOR relay neurons in a form of positive feedback (
Miles and Lisberger, 1981
;
Lisberger and Sejnowski, 1992
,
1993
), or whether vestibular mossy fiber inputs to the flocculus served primarily as a forward side path to the major VOR pathway (
Ito, 1972
,
1982
,
1993a
). Meanwhile, observations about the ventral paraflocculus revealed that the major simple-spike signals driving Purkinje cells were of sensory (visual) origin (
Takemura et al., 2001
) and that this structure provided a parallel pathway to the forward vestibular pathway proposed in the flocculus hypothesis (
Tabata et al., 2002
) (see
Figure 39C
). Hence, there is a dichotomy about the possible role of the flocculus. It may provide either a recurrent positive feedback mechanism (
Figure 39B
) or be a mechanism for converting forward (side path/parallel-pathway) signals to Purkinje cell simple-spike discharges (
Figure 39A
,
C
) and thereby represent an inverse model of the eyeballs (see
Section 12-5
).
