The Cerebellum: Brain for an Implicit Self (29 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

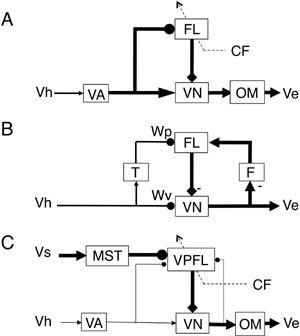
Figure 39. Circuit models of adaptive control of ocular reflexes.
(A) Modifiable side path model for the VOR, assuming a forward path. Based on the ideas of Ito (
1972
,
1974
,
1982
). (B) Recurrent positive feedback model for the VOR proposed by Miles and Lisberger (
1981
) and Lisberger and Sejnowski (
1992
). (C) Parallel pathway model for the OFR developed by Tabata et al. (
2002
). Major pathways in each model are shown by thick lines. Abbreviations: CF, climbing fiber; FL, flocculus; T and F, units inserted to the circuit; MST, medial superior temporal area; OM, motoneurons for extraocular muscles; VA, vestibular afferent system; Ve, eye velocity; Vh, head velocity; Vs, screen velocity; VN, vestibular nuclear neuron; VPFL, ventral paraflocculus. Symbols: arrows indicate fixed synaptic actions or point to output responses; closed circle head, modifiable synapse; square head, inhibitory action of Purkinje cells; broken arrows, climbing fiber pathway; -, inhibition.
Three to four decades have passed since then. We still have no experimental basis, however, for the model-oriented idea that the flocculus receives mossy fiber recurrent collaterals of VOR relay neurons that provide positive feedback of eye velocity signals. Recurrent collaterals are common in cerebellar nuclear neurons, but this cannot be generalized to vestibular nuclei because Deiters neurons in the lateral vestibular nucleus seem to lack recurrent collaterals that project to the cerebellar cortex. In early studies, Deiters neurons were never seen to respond antidromically to stimuli from the cerebellar cortex (
Ito and Yoshida 1966
;
Ito et al., 1969
). This was in contrast to the antidromic responses of cerebellar nuclear neurons to stimuli from their overlying cortex (
Ito et al., 1964
). A side issue is that recurrent collateral input from VOR relay neurons is often assumed to be in accordance with von Holst’s (
1954
) classic concept of “efference copy.” It proposed the existence of an internal centripetally projecting copy of the motor command to cancel the sensory effect of the induced movement (re-afference) (for review, see
Bridgeman, 1995
; Houk et al., 1996). However, no true efference copy appears to accompany the VOR because we see the world moving during the slow phases of vestibular nystagmus. If an efference copy is operative, the world should continue to appear stable during such nystagmus (
Bridgeman, 1995
). This emphasizes the need for caution when suggesting that efference copy is one of the mechanisms of the VOR.
Miles and Lisberger (
1981
) suggested that a memory site is located in vestibular nuclei (
Figure 39B
), as recently confirmed, in addition to parallel fiber-Purkinje cell synapses in the flocculus. A calculation based on their model with two memory sites, however, indicated that parallel fiber-Purkinje cell synapses should undergo an adaptive change in a direction opposite to that expected from the flocculus hypothesis. This led to doubts about the functional role of the flocculus in VOR adaptation. To address this issue, Highstein’s group performed a system identification analysis of Purkinje cells in the monkey flocculus. Their initial measurements, using acute adaptation to a visual-vestibular mismatch, detected a change in Purkinje cell behavior that contradicted the flocculus hypothesis (
Hirata and Highstein, 2001
). However, a later measurement, using chronic adaptation by the wearing of minifying or magnifying lenses, revealed that after their adaptation, Purkinje cells changed their sensitivity to eye position, eye velocity, and head velocity. These combined changes at the Purkinje cell level contributed to a net modulation that was appropriate for supporting the learned VOR gains (
Blazquez et al., 2003
). These analyses, however, were based on a theoretical circuit that assumed the presence of an efference copy pathway. This efference pathway is still a modelistic postulate, as emphasized above. As a result, another open question concerns what occurs if eye velocity signals are derived otherwise: for example, from (1) midline paramedian tract neurons, (2) the conversion of the retinal error signals of climbing fibers, or (3) the input of supplemental signals from other relevant source(s) (
Chapter 10
).
A remarkable finding in the monkey ventral paraflocculus is that during the OFR, simple-spike discharges of Purkinje cells represent an inverse model of an eyeball (
Shidara et al., 1993
;
Yamamoto et al., 1997
;
Gomi et al., 1998
). This model is expressed by a linear summation of eye acceleration, eye velocity, and eye position. The idea and the finding are considered below in relation to the internal model control of voluntary movements (
Chapter 15
, “
Internal Models for Voluntary Motor Control
”). Here, it is considered from the perspective of the feedforward control of reflexes. In an adaptive feedforward control such as the OFR (see earlier), the role of the cerebellum is to tune the overall gain of the control system (equal to -1) at which value the imposed visual target movement is compensated completely by the generated eye movement. This condition is attained when the input/output relationship of the adaptive controller (f) is equated to the output/input relationship of the controlled object (G): that is, f= -1/G; see
Figure 7C
). In the OFR, the ventral paraflocculus should tune f to be equal to 1/G, the reciprocal of the eyeball’s dynamics (G).
A problem about the preceding argument.
The correctness of f = 1/G can be determined if one records from the final output neurons of the adaptive controller, which should be the controller neurons of the OFR. However, in the aforementioned Shidara et al.’s (
1993
) study, the recordings were made in ventral paraflocculus Purkinje cells, and not from the controller neurons, which are located most likely in the vestibular nuclei (
Chapter 10
). Generally speaking, controller neurons are driven by excitatory signals from mossy fibers and inhibitory signals from Purkinje cells, and hence the activity of Purkinje cells should deviate from that of the real controller neurons. Nevertheless, in the Shidara et al. (
1993
) study, pontocerebellar mossy fibers mediating the OFR did not seem to provide any potent excitatory input to vestibular nuclear neurons (see
Figure 38
). Hence, such deviation might have been minimal. It is therefore conceivable that the spike discharges recorded from Purkinje cells by Shidara et al. (
1993
) represented an approximate inverse model of the eyeballs. Similar measurements have also been performed in flocculus Purkinje cells during the vertical OKR (
Mizukoshi et al., 2000
) and horizontal VOR and OKR (
Omata et al., 2000
). The results generally conformed to the inverse model representation of the eyeballs. However, in view of the prominent mossy fiber inputs to the controller neurons of the VOR/OKR (see
Figures 36
and
37
), it is difficult to apply the previous approximation based on the OFR. Recently, recording was made in the Y group of vestibular nuclear neurons (Y neuron) during squirrel monkey’s chronic VOR adaptation (
Blazquez et al. 2006
). In this experiment, Y neurons did not change eye-velocity sensitivity after training, whereas head-velocity sensitivity increased significantly after low-gain but not high-gain training. In contrast, Purkinje cell eye velocity sensitivity changed significantly after low-gain training, and head-velocity sensitivity changed significantly after high-gain training, Apparently, there is no simple reciprocity between Y neurons and Purkinje cells even though they are connected by inhibition. Even though the results still do not allow a simplistic interpretation, it will be valuable to continue these efforts to examine the hypothesis that a microcomplex represents an inverse model of the eyeballs.
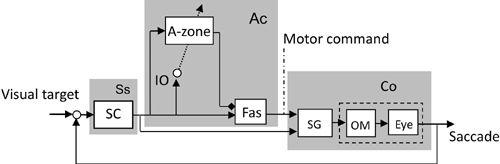
When compared with the previously considered ocular reflexes, the control system structure for saccadic eye movement suggested by experimental data (
Chapter 10
,
Section 8
) is somewhat more complicated. However, if we take the fastigial nucleus as a controller, we need to include the excitatory and inhibitory burst neurons together with the oculomotor neurons, extraocular muscles, and eyeballs as the controlled object (
Figure 40
). This nesting is analogous to the previously presented models for limb withdrawal (
Figure 33
) and eye-blink conditioning (
Figure 41
). The fastigial neurons form a microcomplex with A-zone Purkinje cells, which would function as an adaptive controller, that is similar to the models shown in
Figures 28
and
33
. The superior colliculus may serve for visual information processing just as the MT/MST area functions in OFR control (
Figure 38
).
Figure 40. Control system scheme for a horizontal saccade.
This block diagram is drawn based on the data described in
section 10-8
for saccadic eye movements. Abbreviations: SC, superior colliculus; Fas, fastigial oculomotor region. SG, saccade generator. Note that the illustrated microcomplex (Ac) is of the N-type characterized by the involvement of cerebellar nuclear neurons in the controller and also by the involvement of three units (SG, OM, and Eye) in the controlled object (Co). These features contrast to those in the V-type, which involves vestibular nuclear neurons as the controller and two units in the controlled object (compare with
Figures 36
–
38
).
To model the saccadic system, it is assumed that errors detected via IO climbing fiber signals interact with simple spikes reflecting saccade-generating commands and thereby induce conjunctive LTD (
Schweighofer et al., 1996a
,
b
). The problem of a so-called temporal credit assignment occurs because climbing fiber signals representing consequence errors can reach Purkinje cells only after the arrival of parallel fiber activity related to the saccade-generating command. This problem can be avoided by assuming that parallel fiber activity results in long-lasting chemical signal transduction effects, in particular, the generation of IP
3
(
Chapter 7
, “
Conjunctive Long-Term Depression (LTD)
”). In a recent model of saccadic adaptation, Fujita (
2005
) assumed that a consequence error detected at the end of the first saccade was converted to a motor command in order to initiate a corrective saccade and that the corrective command triggered climbing fiber activity. In spite of the fact that such climbing fiber activity arises after the first saccade, it may still induce conjunctive LTD in the parallel fiber-Purkinje cell synapses involved in the first saccade. The reason is that parallel fiber-evoked signal transduction in Purkinje cells lasts long enough to interact with the climbing fiber signals that arrive somewhat later. The conjunctive LTD so induced modifies the first saccade adaptively to “catch up with” the second saccade. When a large, realistic cerebellar neuronal network was incorporated into a saccade generator model, the complex spatiotemporal behavior of the neuronal subpopulations implicated in adaptive saccadic control was modeled in a manner that was consistent with the experimental data (
Schweighofer et al., 1996b
).
Similar control system models can be applied to somatic reflexes. Deiters neurons, via the lateral vestibulospinal tract, function as the controller of the controlled object provided by the segmental circuit for extensor muscles (
Figure 32
). In this system, the peripheral proprioceptive and cutaneous sensory signals are sent to Deiters neurons via spinocerebellar tracts. Deiters neurons, in turn, send descending signals to excite extensor motoneurons and inhibit their inhibitory interneurons. As a result, a limb can remain extended during a standing posture or during the supporting (stance) phase of stepping. Attached to this long loop reflex, the B-zone receives mossy fiber afferents from the spinocerebellar tracts and, in turn, projects Purkinje cell axons directly to Deiters neurons. Climbing fiber responses occur in B-zone Purkinje cells when an error is sensed (
Chapter 10
).
