Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine (5 page)
Read Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine Online
Authors: Marc Sabatine
Tags: #Medical, #Internal Medicine

BOOK: Pocket Medicine: The Massachusetts General Hospital Handbook of Internal Medicine
5.2Mb size Format: txt, pdf, ePub
• Radionuclide: ≥1 lg or ≥2 mod. reversible defects, transient LV cavity dilation, ↑ lung uptake
Myocardial viability (
Circ
2008;117:103;
Eur Heart J
2011;31:2984 & 2011;32:810)
• Goal: identify hibernating myocardium that could regain fxn after revascularization • Options:
MRI
(Se ~95%, Sp ~85%),
PET
(Se ~90%, Sp ~65%),
dobutamine stress
echo
(Se ~80%, Sp ~80%);
SPECT/rest-redistribution
(Se ~85%, Sp ~70%)
In Pts w/ LV dysfxn, viabil. doesn’t predict ↑ CABG benefit vs. med Rx (
NEJM
2011;364:1617)
CT & MR coronary angio (
NEJM
2008;369:2324;
Circ
2010;121:2509;
Lancet
2012;379:453)
• Image quality best at slower & regular HR (? give bB if possible, goal HR 55–60) • Calcium generates artifact for CT angiography • MRI: angiography, perfusion, LV fxn, enhancement (early = microvasc obstr; late = MI)
Coronary artery calcium score
(CACS;
Circ
2010;122:e584;
NEJM
2012;366:294;
JAMA
2012;308:788)
• Quantifies extent of calcium; thus
estimates
plaque burden (but
not
% coronary stenosis) • ? Risk strat. (<100 = low; >300 = high) in asx Pts w/ intermed risk (10–20% 10-y risk) • ? Value as screening test to r/o CAD in sx Pt (CACS <100 → 3% probability of signif CAD; but interpretation affected by age, gender)
CORONARY ANGIOGRAPHY AND REVASCULARIZATION
Indications for coronary angiography in stable CAD or asx Pts
• CCS class III–IV angina despite medical Rx or angina + systolic dysfxn • High-risk stress test findings (see prior topic) • Uncertain dx after noninvasive testing (& compelling need to determine dx), occupational need for definitive dx (eg, pilot) or inability to undergo noninvasive testing • Systolic dysfxn with unexplained cause • Survivor of SCD, polymorphic VT, sustained monomorphic VT
• Suspected spasm or nonatherosclerotic cause of ischemia (eg, anomalous coronary)
Precath checklist
• Document peripheral arterial exam (radial, femoral, DP, PT pulses; bruits); NPO >6 h • ✓ CBC, PT, & Cr; give IVF (± bicarb, ± acetylcysteine; see “CIAKI”); blood bank sample • ASA 325 mg × 1; consider clopi 600 mg ≥2–6 h before PCI or, if ACS, ticagrelor pre-or peri-PCI or prasugrel peri-PCI; cangrelor (IV P2Y
12
inhib) ↓ peri-PCI ischemic events vs. clopi w/o preload (
NEJM
2013;368:1303); consider statin preRx (
Circ
2011;123:1622)
Coronary revascularization in stable CAD (
Circ
2011;124:e574)
• Optimal med Rx (
OMT
) should be initial focus if stable, w/o critical anatomy, & w/o ↓ EF
•
PCI
: ↓ angina more quickly c/w OMT; does
not
↓ D/MI (
NEJM
2007;356:1503); in Pts w/ ≥1 stenosis w/ FFR ≤0.8 (see below), ↓ urg revasc c/w OMT (
NEJM
2012;367:991); may be noninferior to CABG in unprotected left main dis. (
NEJM
2011;364:1718) •
CABG
: in older studies, ↓ mort. c/w OMT if 3VD, LM, 2VD w/ critical prox LAD, esp. if ↓ EF; more recently, if EF <35% ↓ CV death vs. OMT (
NEJM
2011;364:1607) insufficient evidence to support routine viability assessment (
NEJM
2011;364:1617) in diabetics w/ ≥2VD, ↓ D/MI, but ↑ stroke c/w PCI (
NEJM
2012;367:2375) • If revasc deemed necessary,
PCI
if limited # of discrete lesions, nl EF, no DM, poor operative candidate;
CABG
if extensive or diffuse disease, ↓ EF, DM or valvular disease; if 3VD/LM: CABG ↓ D/MI & revasc but trend toward ↑ stroke c/w PCI (
Lancet
2013;381:629); SYNTAX score II helps identify Pts who benefit most from CABG (
Lancet
2013;381:639)
PCI
•
Balloon angioplasty (POBA)
: effective, but c/b dissection & elastic recoil & neointimal hyperplasia → restenosis; now reserved for small lesions & ? some SVG lesions •
Bare metal stents (BMS)
: ↓ elastic recoil → 33–50% ↓ restenosis & repeat revasc (to ~10% by 1 y) c/w POBA; requires ASA lifelong & P2Y
12
inhib × ≥4 wk •
Drug-eluting stents (DES)
: ↓ neointimal hyperplasia → ~75% ↓ restenosis, ~50% ↓ repeat revasc (to <5% by 1 y), no ↑ D/MI c/w BMS (
NEJM
2013;368:254); next generation DES may ↓ repeat revasc & stent thrombosis; require P2Y
12
inhib ≥1 y (
Circ
2007;115:813) • Radial access ↓ vasc. complic. vs. femoral, but no ∆ D/MI/CVA (
Lancet
2011;377:1409) • Fractional flow reserve [FFR; ratio of max flow (induced by IV or IC adenosine) distal vs. proximal to a stenosis] guided PCI (<0.8) → ↓ # stents & ↓ D/MI/revasc (
NEJM
2009;360:213)
Post-PCI complications
• Postprocedure ✓ vascular access site, distal pulses, ECG, CBC, Cr •
Bleeding
hematoma/overt bleeding:
manual compression
, reverse/stop anticoag
retroperitoneal bleed:
may p/w ↓ Hct ± back pain; ↑ HR & ↓ BP late; Dx w/ abd/pelvic CT (I
–
); Rx: reverse/stop anticoag (d/w interventionalist), IVF/PRBC/plts as required
if bleeding uncontrolled, consult performing interventionalist or surgery
•
Vascular damage
(~1% of dx angio, ~5% of PCI;
Circ
2007;115:2666)
pseudoaneurysm: triad of pain, expansile mass, systolic bruit; Dx: U/S; Rx (if pain or >2 cm): manual or U/S-directed compression, thrombin injection or surgical repair
AV fistula: continuous bruit; Dx: U/S; Rx: surgical repair
LE ischemia (emboli, dissection, clot): cool, mottled extremity, ↓ distal pulses; Dx: pulse volume recording (PVR), angio; Rx: percutaneous or surgical repair
•
Peri-PCI MI
: >5× ULN of Tn/CK-MB + either sx or ECG/angio Δs; Qw MI in <1%
•
Renal failure
: contrast-induced manifests w/in 24 h, peaks 3–5 d (see “CIAKI”) •
Cholesterol emboli syndrome
(typically in middle-aged & elderly and w/ Ao atheroma)
renal failure (late and progressive, eos in urine); mesenteric ischemia (abd pain, LGIB, pancreatitis); intact distal pulses but livedo pattern and toe necrosis
•
Stent thrombosis
: mins to yrs after PCI, typically p/w AMI. Due to mech prob. (stent underexpansion or unrecognized dissection, typically presents early) or
d/c of antiplt Rx
(esp. if d/c both ASA & P2Y
12
inhib;
JAMA
2005;293:2126). Risk of late stent thrombosis may be higher with DES than BMS (
JACC
2006;48:2584).
•
In-stent restenosis
: mos after PCI, typically p/w gradual ↑ angina (10% p/w ACS). Due to combination of elastic recoil and neointimal hyperplasia; ↓ w/ DES vs. BMS.
ACUTE CORONARY SYNDROMES
Ddx (causes of myocardial ischemia/infarction other than atherosclerotic plaque rupture)
• Nonatherosclerotic coronary artery disease
Spasm: Prinzmetal’s variant, cocaine-induced (6% of CP + cocaine use r/i for MI)
Dissection: spontaneous (vasculitis, CTD, pregnancy), aortic dissection with retrograde extension (usually involving RCA → IMI) or mechanical (catheter, surgery, trauma)
Embolism: endocarditis, prosthetic valve, mural thrombus, AF, myxoma; thrombosis
Vasculitis: Kawasaki syndrome, Takayasu arteritis, PAN, Churg-Strauss, SLE, RA
Congenital: anomalous origin from aorta or PA, myocardial bridge (intramural segment)
• Fixed CAD but ↑ myocardial O
2
demand (eg, ↑ HR, anemia, AS) → “demand” ischemia • Myocarditis; Takatsubo/stress CMP; toxic CMP; cardiac contusion
Clinical manifestations (
JAMA
2005;294:2623)
•
Typical angina
: retrosternal pressure/pain/tightness ± radiation to neck, jaw or arms
precip. by exertion, relieved by rest or NTG; in ACS, new-onset, crescendo or at rest
•
Associated symptoms
: dyspnea, diaphoresis, N/V, palpitations or lightheadedness • Many MIs (~20% in older series) are initially unrecognized b/c silent or atypical sx
Physical exam
• Signs of ischemia: S
4
, new MR murmur 2° pap. muscle dysfxn, paradoxical S
2
, diaphoresis • Signs of heart failure: ↑ JVP, crackles in lung fields,S
3
, HoTN, cool extremities • Signs of other areas of atherosclerotic disease: carotid or femoral bruits, ↓ distal pulses
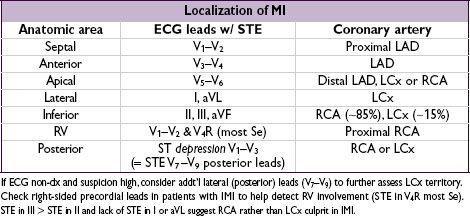
Diagnostic studies
•
ECG
: ST ↓/↑, TWI, new LBBB, hyperacute Tw. Qw/PRWP may suggest prior MI, ∴ CAD ✓ ECG w/in 10 min of presentation, with any Δ in sx and at 6–12 h; compare w/ baseline
dx of STEMI if old LBBB: ≥1 mm STE concordant w/ QRS (Se 73%, Sp 92%), STD ≥1 mm V
1
–V
3
(Se 25%, Sp 96%) or STE ≥5 mm discordant w/ QRS (Se 31%, Sp 92%)
Other books
Little Black Girl Lost by Keith Lee Johnson
But Enough About You: Essays by Christopher Buckley
Reign (An Unfortunate Fairy Tale Book 4) by Chanda Hahn
Transit by Abdourahman A. Waberi
Shattered by Jay Bonansinga
The Curve Ball by J. S. Scott
Three Little Maids by Patricia Scott
The Red Thirst by Benjamin Hulme-Cross
Conman by Richard Asplin
Snare by Katharine Kerr