Make: Electronics (12 page)
Authors: Charles Platt

This is seriously old-school—but I want you to try it anyway, because anyone who wants to get a feel for electricity should see how easy it is to extract it from everyday objects around us. Plus, if you use enough lemons, you just
might
generate enough voltage to power an LED.
The basic components of a battery are two metal electrodes immersed in an electrolyte. I won’t define these terms here (they’re explained in the following section “Theory: The nature of electricity”). Right now all you need to know is that lemon juice will be your electrolyte, and copper and zinc will be your electrodes. A penny provides the necessary copper, as long as it is fairly new and shiny. Pennies aren’t solid copper anymore, but they are still copper-plated, which is good enough.
To find some metallic zinc, you will have to make a trip to a hardware store, where you should ask for roofing nails. The nails are zinc-plated to prevent them from rusting. Small metal brackets or mending plates also are usually zinc-plated. They should have a slightly dull, silvery look. If they have a mirror-bright finish, they’re more likely to be nickel-plated.
Cut a lemon in half, set your multimeter so that it can measure up to 2 volts DC, and hold one probe against a penny while you hold the other probe against a roofing nail (or other zinc-plated object). Now force the penny and the nail into the exposed juicy interior of the lemon, as close to each other as possible, but not actually touching. You should find that your meter detects between 0.8 volts and 1 volt.
You can experiment with different items and liquids to see which works best. Immersing your nail and penny in lemon juice that you have squeezed into a shot glass or egg cup may enhance the efficiency of your battery, although you’ll have a harder time holding everything in place. Grapefruit juice and vinegar will work as substitutes for lemon juice.
To drive a typical LED, you need more than 1 volt. How to generate the extra electrical pressure? By putting batteries in series, of course. In other words, more lemons! (Or more shot glasses or egg cups.) You’ll also need lengths of wire to connect multiple electrodes, and this may entail skipping ahead to
Chapter 2
, where I describe how to strip insulation from hookup wire. Figures 1-71 and 1-72 show the configuration.
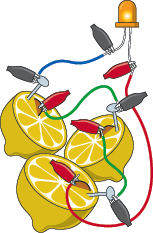
Figure 1-71.
A three-lemon battery. Don’t be too disappointed if the LED fails to light up. The lemons have a high electrical resistance, so they can’t deliver much current, especially through the relatively small surface area of the nails and the pennies. However, the lemon battery does generate voltage that you can measure with your meter.

Figure 1-72.
Bottled lemon juice seems to work just as well as fresh lemon juice. I cut the bottoms off three paper cups, inserted a galvanized bracket into each, and used heavyweight stranded copper wire to make the positive electrodes
If you set things up carefully, making sure than none of the electrodes are touching, you may be able to illuminate your LED with two or three lemon-juice batteries in series. (Some LEDs are more sensitive to very low currents than others. Later in the book I’ll be talking about very-low-current LEDs. If you want your lemon-juice battery to have the best chance of working, you can search online for low-current LEDs and buy a couple.)
Theory
The nature of electricity
To understand electricity, you have to start with some basic information about atoms. Each atom consists of a nucleus at the center, containing protons, which have a positive charge. The nucleus is surrounded by electrons, which carry a negative charge.
Breaking up the nucleus of an atom requires a lot of energy, and can also liberate a lot of energy—as happens in a nuclear explosion. But persuading a couple of electrons to leave an atom (or join an atom) takes very little energy. For instance, when zinc reacts chemically with an acid, it can liberate electrons. This is what happens at the zinc electrode of the chemical battery in
Experiment 5
.
The reaction soon stops, as electrons accumulate on the zinc electrode. They feel a mutual force of repulsion, yet they have nowhere to go. You can imagine them like a crowd of hostile people, each one wanting the others to leave, and refusing to allow new ones to join them, as shown in Figure 1-73.

Figure 1-73.
Electrons on an electrode have a bad attitude known as mutual repulsion.
Now consider what happens when a wire connects the zinc electrode, which has a surplus of electrons, to another electrode, made from a different material, that has a shortage of electrons. The electrons can pass through the wire very easily by jumping from one atom to the next, so they escape from the zinc electrode and run through the wire, propelled by their great desire to get away from each other. See Figure 1-74. This mutual force of propulsion is what creates an electrical current.
Now that the population of electrons on the zinc electrode has been reduced, the zinc-acid reaction can continue, replacing the missing electrons with new ones—which promptly imitate their predecessors and try to get away from each other by running away down the wire. The process continues until the zinc-acid reaction grinds to a halt, usually because it creates a layer of a compound such as zinc oxide, which won’t react with acid and prevents the acid from reacting with the zinc underneath. (This is why your zinc electrode may have looked sooty when you pulled it out of the acidic electrolyte.)
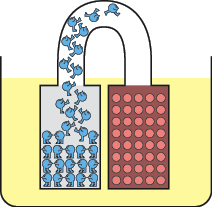
Figure 1-74.
As soon as we open up a pathway from a zinc electrode crowded with electrons to a copper electrode, which contains “holes” for the electrons, their mutual repulsion makes them try to escape from each other to their new home as quickly as possible.
This description applies to a “primary battery,” meaning one that is ready to generate electricity as soon as a connection between its terminals allows electrons to transfer from one electrode to the other. The amount of current that a primary battery can generate is determined by the speed at which chemical reactions inside the battery can liberate electrons. When the raw metal in the electrodes has all been used up in chemical reactions, the battery can’t generate any more electricity and is dead. It cannot easily be recharged, because the chemical reactions are not easily reversible, and the electrodes may have oxidized.
In a rechargeable battery, also known as a secondary battery, a smarter choice of electrodes and electrolyte does allow the chemical reactions to be reversed.
How much current is being generated in your lemon battery? Set your meter to measure milliamps, and connect it between the nail and the penny. I measured about 2mA, but got 10mA when I used some #10 stranded copper wire instead of a penny and a large mending plate instead of a roofing nail, immersed in a cup of grapefruit juice. When a larger surface area of metal makes better contact with the electrolyte, you get a greater flow of current. (Don’t ever connect your meter to measure amps directly between the terminals of a real battery. The current will be too high, and can blow the fuse inside your meter.)
What’s the internal resistance of your lemon? Put aside the copper and zinc electrodes and insert your nickel-plated meter probes into the juice. I got a reading of around 30K when both probes were in the same segment of the lemon, but 40K or higher if the probes were in different segments. Is the resistance lower when you test liquid in a cup?
Here are a couple more questions that you may wish to investigate. For how long will your lemon battery generate electricity? And why do you think your zinc-plated electrode becomes discolored after it has been used for a while?
Electricity is generated in a battery by an exchange of ions, or free electrons, between metals. If you want to know more about this, check the section “Theory: The nature of electricity” on the previous page.
Cleanup and Recycling
The hardware that you immersed in lemons or lemon juice may be discolored, but it is reusable. Whether you eat the lemons is up to you.
Background
Positive and negative
If electricity is a flow of electrons, which have a negative charge, why do people talk as if electricity flows from the positive terminal to the negative terminal of a battery?
The answer lies in a fundamental embarrassment in the history of research into electricity. For various reasons, when Benjamin Franklin was trying to understand the nature of electric current by studying phenomena such as lightning during thunderstorms, he believed he observed a flow of “electrical fluid” from positive to negative. He proposed this concept in 1747.
In fact, Franklin had made an unfortunate error that remained uncorrected until after physicist J. J. Thomson announced his discovery of the electron in 1897, 150 years later. Electricity actually flows from an area of greater negative charge, to some other location that is “less negative”—that is, “more positive.” In other words, electricity is a flow of negatively charged particles. In a battery, they originate from the negative terminal and flow to the positive terminal.
You might think that when this fact was established, everyone should have discarded Franklin’s idea of a flow from positive to negative. But when an electron moves through a wire, you can still think of an equal positive charge flowing in the opposite direction. When the electron leaves home, it takes a small negative charge with it; therefore, its home becomes a bit more positive. When the electron arrives at its destination, its negative charge makes the destination a bit less positive. This is pretty much what would happen if an imaginary positive particle traveled in the opposite direction. Moreover, all of the mathematics describing electrical behavior are still valid if you apply them to the imaginary flow of positive charges.
As a matter of tradition and convenience we still retain Ben Franklin’s erroneous concept of flow from positive to negative, because it really makes no difference. In the symbols that represent components such as diodes and transistors, you will actually find arrows reminding you which way these components should be placed—and the arrows all point from positive to negative, even though that’s not the way things really work at all! Ben Franklin would have been surprised to learn that although most lightning strikes occur when a negative charge in clouds discharges to neutralize a positive charge on the ground, some forms of lightning are actually a flow of electrons from the negatively charged surface of the earth, up to a positive charge in the clouds. That’s right: someone who is “struck by lightning” may be hurt by
emitting
electrons rather than by receiving them, as shown in Figure 1-75.
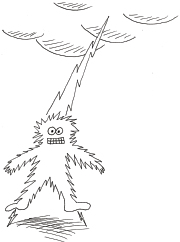
Figure 1-75.
In some weather conditions, the flow of electrons during a lightning strike can be from the ground, through your feet, out of the top of your head, and up to the clouds. Benjamin Franklin would have been surprised.
