The Flamingo’s Smile (3 page)
Read The Flamingo’s Smile Online
Authors: Stephen Jay Gould

The first extensive and explicit commentary had been offered in 1681 by Nehemiah Grew, the great English naturalist (known primarily for his early microscopical studies of plants). Cataloguing the collections of the Royal Society—in his
Musaeum Regalis Societatis, or a catalogue and description of the natural and artificial rarities belonging to the Royal Society and presented at Gresham Colledge, whereunto is subjoined the comparative anatomy of stomachs and guts
—he encountered a lone flamingo (see figure) and stated: “that wherein he is most remarkable, is his bill.” Grew suspected that the oddities of the bill would all be resolved if the upper beak moved against a stationary lower jaw. He stated that the “shape and bigness of the upper beak (which here, contrary to what it is in all other birds that I have seen, is thinner and far less than the nether) speaks it to be more fit for motion and to make the appulse and the nether to receive it.”
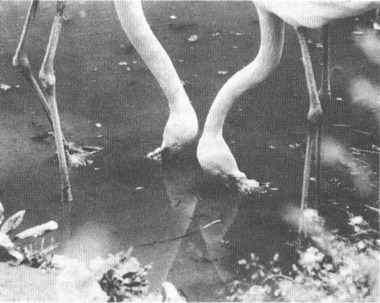
Flamingos in their characteristic feeding pose—upside down.
PHOTO BY D. PURCELL
.
The question was not fully resolved until Jenkin published her comprehensive paper in 1957—affirming with hard data the suspicions and good judgment of Menippus, Grew, and Buffon. In fact, flamingos (along with many other birds) have developed a highly mobile ball and socket joint between upper and lower jaws. The beaks therefore have great mobility, and each can move independently. In preening, either the upper or lower jaw may be opened and operated against the other. But, in feeding, the upper jaw usually drops and raises against a stationary lower jaw—just as the great naturalists had always expected.
The flamingo’s flip-flop is complete and comprehensive—in form
and
motion. The shapes are overturned by bending, the sizes reversed, the slotting inverted, the buttressing transposed. The action, too, is topsy-turvy. A peculiar reversal in behavior has engendered a complex inversion of form. Evolution as adaptation to particular modes of life—Darwin’s vision—gains strength from an extreme test imposed by life upside down.
But do flamingos just provide a funny example, or do they symbolize a generality? What about other creatures that live upside down? Consider another animal of shallow West Indian waters, the inverted jellyfish,
Cassiopea xamachana
(the unorthodox trivial name honors the Native American designation for the island of Jamaica).
Cassiopea
is an unconventional jellyfish in many ways. It grows neither marginal tentacles nor central mouth. Instead, eight fleshy and complexly branched “oral arms” (so called because each contains a separate mouth) emerge from a short and stout central stalk, itself attached to a usual jellyfish umbrella with a difference (see figure—a reproduction of the classical lithograph from Mayer’s 1910 monograph,
Medusae of the World
). The oral arms are crammed with symbiotic algal cells, a possible adaptive impetus for their elaborate branching (to provide light-capturing surfaces for the photosynthetic symbionts). Each oral arm harbors about forty oral vesicles—hollow sacs connected with the feeding canals and containing bags of nematocysts, or stinging cells, at their tips. The vesicles shoot their nematocysts at prey (mostly small crustaceans) in strings of mucus; the strings with their attached and paralyzed victims are then pulled into the oral mouths. (Yes, I was as amused as some of you by the redundant “oral mouth”—the zoological equivalent of pizza pie or AC current. This clumsy phrase arises as unfelicitous fallout from a prior decision to call the appendages “oral arms”—as a shortcut for “mouths of the oral arms.”)
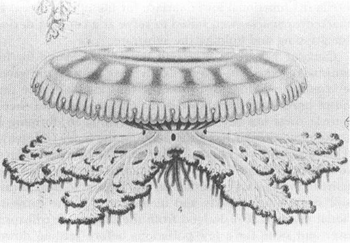
Cassiopea xamachana
. Note concavity of bell’s upper surface and the raised muscular ring. Figure reproduced as presented (in the ecologically wrong right-side-up position).
FROM MAYER.
1910.
REPRINTED FROM NATURAL HISTORY
.
Cassiopea
’s unusual anatomy matches its unconventional orientation and style of life. Ordinary, self-respecting jellyfish swim actively with their umbrellas uppermost and their arms and tentacles below.
Cassiopea
lies stationary on the bottom of shallow ponds and coastal areas—upside down. The top of its umbrella hugs the sediment and the oral arms wave above, waiting for small crustacea to enter their orbit. Sailors at Fort Jefferson in the Tortugas, where
Cassiopea
lined the docks, called them “moss cakes.” (Since
Cassiopea
can give a nasty sting, and since men in blue usually spice their language to match the stimulus, I wonder what the sailors really called them. But Mr. H.F. Perkins, writing in 1908 on the anatomy of
Cassiopea
, didn’t choose to tell us.)
The umbrella of
Cassiopea
recalls the flamingo’s jaw in its adaptation to reversed life. The umbrella’s upper surface is smoothly convex in ordinary jellyfish, as hydrodynamic efficiency dictates. But the upper surface of
Cassiopea
’s umbrella (its functional lower surface for life upside down) is markedly
concave
—well suited to serve as a cupping device for gripping and holding the substrate.
Cassiopea
has made a second intriguing change for its unusual reversed life. Most jellyfish move through the water by contracting rings of concentric muscles that circle the outer portion of the umbrella. In
Cassiopea
, one of these muscle rings has been raised and accentuated, forming a continuous circular band surrounding the inner concavity. This raised rim operates together with the concave surface to form an efficient suction cup that keeps the “head” of this jellyfish in its proper position on the bottom. (
Cassiopea
can still swim, albeit weakly and inefficiently, in the conventional manner. If dislodged from the bottom, it will turn over and swim for a few pulsations before settling down again on its head.) Some scientists have also suggested that the pulsating contractions of the concentric muscles, ordinarily used in swimming, serve other important functions in
Cassiopea
’s attached, upside-down position—maintaining connection with the substrate by pushing the animal down and moving water currents with potential prey towards the oral arms. But these reasonable proposals have not been properly tested.
Thus, flamingos and
Cassiopea
—two animals that could scarcely differ more in design and evolutionary history—share the common feature of feeding upside down. As a general message amidst the particulars, they have both redesigned conventional anatomy to match reversed life style. The flamingo’s upper bill has changed radically—in size, shape, and motion—to look and work like the lower beak of most birds. The structural top of
Cassiopea
’s umbrella has inverted its shape, all the better to work properly as an ecological bottom.
Adaptation has a wonderful power to alter an anatomical design, widespread and stable among thousands of species, for the reversed requirements of an odd life style assumed by one or a few aberrant forms. Yet, we should not conclude that Darwinian adaptation to local environments has unconstrained power to design theoretically optimum shapes for all situations. Natural selection, as a historical process, can only work with material available—in these cases, the conventional designs evolved for ordinary life. The resulting imperfections and odd solutions, cobbled together from parts on hand, record a process that unfolds in time from unsuited antecedents, not the work of a perfect architect creating
ab nihilo. Cassiopea
co-opts a band of muscles ordinarily used in swimming and forms a raised rim to grasp the substrate. Flamingos bend their bill in a curious hump as the only topological solution to a new orientation.
These adaptations to life upside down are not just funny facts. They help us to comprehend the solution to a major, and classical, dilemma in evolutionary theory (hence my decision to unite them in this essay). We can easily understand how flamingos and
Cassiopea
work; their unusual features do fit them for their unconventional lives. But how do these odd structures arise if evolution must proceed through intermediate steps (no one will seriously suggest that the first proto-flamingo turned its head upside down and then produced offspring with a complete set of complex adaptations to reversed life).
In pre-Darwinian years of the early nineteenth century, when evolution was new, and when early exponents of such a radical idea were trying to work out its ramifying implications, two schools emerged and carried out an interesting (and largely forgotten) struggle until Darwin resolved their debate. Both sides admitted the good fit that usually exists between form and function—adaptation in its static, non-historical meaning. Structuralists, like Etienne Geoffroy Saint-Hilaire argued that form must change first and then find a function. Functionalists, like Jean Baptiste Lamarck, held that organisms must first adopt a different mode of life to trigger some sort of pressure for a subsequently altered form.
The nature of this “pressure” inspired another famous (and better remembered, but no more important) debate. Lamarck held that organisms respond creatively to the needs imposed by their environments and then pass the resulting changes directly to offspring—“inheritance of acquired characters” in the usual jargon. Darwin argued that environments do not impose their adaptive requirements directly. Rather, those organisms that vary, by good fortune, in directions better suited to local environments leave more surviving offspring by a process of natural selection.
Since Darwin won this argument about the nature of signals that pass from environment to organism, Lamarck has been eclipsed and still, despite many efforts by historians to set the record straight, suffers from an imposed reputation as a loser not to be taken seriously for any of his ideas.
But Lamarck had the right answer (the same as Darwin’s) to the larger dispute between structuralists and functionalists. (He only proposed the wrong mechanism for how environment gets its message to organisms.) Geoffroy’s structuralist solution poses an obvious dilemma. If structure changes first, according to unknown “laws of form,” and then finds the environment best suited to its altered state, how can precise adaptation arise? We might allow that some very basic and general changes could precede any functional meaning or advantage—an animal might, for example, get larger and then exploit the inherent advantages of increased size. But can we seriously believe that something so complex, so multifarious, and so intimately suited for an unusual ecology as the flamingo’s bill might arise before the fact and without relationship to its usefulness—permitting the flamingo to discover only later how nicely such a beak worked upside down?
Lamarck’s functionalist solution has an elegant simplicity accepted by nearly all evolutionists today (but usually attributed to Darwin, who also supported it. However much I revere Darwin, I want to advance a plea for recognizing this basic principle as Lamarck’s primary contribution. It does not appear as an incidental footnote in Lamarck’s
Philosophie zoologique
of 1809, but as a central theme of his book. Lamarck knew exactly what he was arguing and why.). Lamarck simply recognized that change of behavior must precede alteration of form. An organism enters a new environment with its old form suited to other styles of life. The behavioral innovation establishes a discordance between new function and inherited form—an impetus to change (by creative response and direct inheritance for Lamarck, by natural selection for Darwin). The protoflamingo first inverts its normal bill—and it doesn’t work very well. The proto-
Cassiopea
turns over, but its convex umbrella doesn’t clutch the substrate. Lamarck wrote:
It is not the shape either of the body or its parts, which gives rise to the habits of animals and their mode of life; but it is, on the contrary, the habits, mode of life, and all the other influences of the environment, which have in course of time built up the shape of the body and of the parts of animals.
The direct evidence for Lamarck’s solution cannot emerge from such “completed” adaptations as the flamingo’s beak or
Cassiopea
’s umbrella—though the inference even here becomes quite compelling (for why should flamingos, uniquely among birds, develop such a peculiar beak if not to exploit their chosen, odd environment). We must catch the process at its beginning stages—by finding upside down animals that have already altered their behavior, but not their form.
