The Flamingo’s Smile (22 page)
Read The Flamingo’s Smile Online
Authors: Stephen Jay Gould

This theme of greater difference between mass extinctions and “normal” times gained strength and refinement in several papers presented at Indianapolis. Jack Sepkoski, a former student of mine now flourishing mightily at the University of Chicago, has spent years compiling the most consistent and complete data set ever developed for extinctions—a listing at the family level that includes everything from protozoans to mammals. With these data, we have finally achieved a basis for the fine-scaled consideration of quantitative patterns in extinction that this second strategy demands. (Good science may require genius and imagination, as these essays so often emphasize, but never forget that new conclusions are the fruit of hard empirical labor as well—otherwise, highfalutin thought is so much waffling.)
Using the Sepkoski data, Raup and Sepkoski have now identified a striking cyclicity in mass extinctions for 225 million years since the great Permian dying. Every 26 million years, with eight hits and just two apparent misses (a pattern too regular and striking to dismiss as accidental on statistical grounds), we find a peak of mass extinction; all previously identified disasters lie right on the highs of this 26-million-year cycle. What cause could yield a periodicity so regular, yet so widely spaced? If we understand geology aright, no purely internal process of climate, volcanism, or plate tectonics cycles so regularly with such a long period. Raup and Sepkoski therefore speculate that some astronomical cycle must be implicated—a solar or galactic phenomenon, although for the moment, we have no idea what.
*
If disasters are so frequent and caused by events so utterly beyond an organism’s control or anticipation (how can populations track a 26-million-year cycle?), and if these coordinated dyings shape life’s pattern so fundamentally, then mass extinction is not ordinary death extrapolated.
David Jablonski, a paleobiologist from the University of Arizona at Tucson, then added two cogent arguments to emphasize the abruptness and the different character of mass extinctions. For abruptness, Jablonski noted that the raw data of mass extinctions often include a long period of apparently slow and steady decline among groups that crash more profoundly at the peak itself. These slow declines have long been interpreted as a sign of continuity between normal and mass extinction. But are they real or an artifact of our imperfect geological record?
For more than one hundred years, geologists have sought terrestrial agents to associate with mass extinction. The litany is long, yet all but one have failed—mountain building, volcanism, fluctuations in temperature, to name just a few old and unsuccessful favorites. Falling sea level provides the one good correlation (and the 26-million-year-cycle theorists had better take it into account). Most mass extinctions are preceded by a marked regression of sea level.
Falling sea level may participate as a cause of extinction (our fossil record is strongly biased toward shallow-water marine invertebrates), but it also imposes an obvious artifact upon our data. As sea level falls, fewer sedimentary rocks form to hold the fossils of limited oceans. Perhaps the slow decline that precedes most mass extinctions only records the decreasing volume of rock available for finding fossils, not a true and gradual decrease presaging the later peak.
Jablonski devised a clever method to measure the potential artifact. Some forms disappear from the record as sea level falls, only to come back again when seas return to deposit more rocks after the mass extinction itself. These temporary losses must record an artificial effect of falling seas and decreasing amounts of fossiliferous rock. Jablonski refers to these reappearing groups as “Lazarus taxa.”
By counting the number of Lazarus taxa that disappear before, but reappear after, a mass extinction, Jablonski can estimate how much of a measured slow decline before a mass extinction might be the artificial result of less available rock for finding fossils, and how much must record a real and gradual event connecting peaks of mass extinction with normal times before.
In some cases, subtraction of the Lazarus taxa still leaves a residue of slow disappearance, and the pattern must be real (decline of ammonites before the Cretaceous extinction, for example). But for many Cretaceous groups, a measured slow decline can be attributed entirely to the artifact of decreasing available rock. Thus, the Cretaceous extinction, and others as well, may be more abrupt than we have previously realized. The case for an extraterrestrial agent gains strength. Mass extinction is something quick and special.
Jablonski then examined the behavior of groups during normal times and during episodes of mass extinction to see if he could detect consistent differences that might accentuate the special character of mass extinctions. He found some intriguing disparities. Some branches of the evolutionary tree contain many species either because new species form easily or because they resist extinction once they arise. Jablonski calls these branches “species-rich clades” as opposed to “species-poor clades,” or groups that never contain many species.
During normal times, species-rich clades tend to increase their numbers of species continually—and to win increasing numerical advantage over species-poor clades. The environments of normal times must encourage either rapid speciation or persistence thereafter. But why, then, don’t species-rich clades take over the biosphere entirely? Jablonski finds that these same species-rich clades fare worse than species-poor clades during mass extinctions. The individual species in species-poor clades have wider geographic ranges and broader ecological tolerances than the narrow-niched taxa of species-rich clades. This geographic and ecological breadth probably protects such species in the extreme environments that mass extinction must generate. These same features of breadth may cut down the rate of speciation in normal times (fewer opportunities for isolation and exploitation of new environments), thus rendering such groups species-poor.
This contrary behavior of species-rich clades in normal and catastrophic times preserves a balance that permits both species-rich and species-poor clades to flourish throughout life’s history. More important in our context, this distinction emphasizes the qualitative difference between normal times and catastrophic zaps. Mass extinctions are not simply more of the same. They affect various elements of the biosphere in a distinctive manner, quite different from the patterns of normal times.
As we survey the history of life since the inception of multicellular complexity in Ediacaran times (see essay 16), one feature stands out as most puzzling—the lack of clear order and progress through time among marine invertebrate faunas. We can tell tales of improvement for some groups, but in honest moments we must admit that the history of complex life is more a story of multifarious variation about a set of basic designs than a saga of accumulating excellence. The eyes of early trilobites, for example, have never been exceeded for complexity or acuity by later arthropods. Why do we not find this expected order?
Perhaps the expectation itself is faulty, a product of pervasive, progressivist bias in Western thought and never a prediction of evolutionary theory. Yet, if natural selection rules the world of life, we should detect some fitful accumulation of better and more complex design through time—amidst all the fluctuations and backings and for things that must characterize a process primarily devoted to constructing a better fit between organisms and changing local environments. Darwin certainly anticipated such progress when he wrote:
The inhabitants of each successive period in the world’s history have beaten their predecessors in the race for life, and are, insofar, higher in the scale of nature; and this may account for that vague yet ill-defined sentiment, felt by many paleontologists, that organization on the whole has progressed.
I regard the failure to find a clear “vector of progress” in life’s history as the most puzzling fact of the fossil record. But I also believe that we are now on the verge of a solution, thanks to a better understanding of evolution in
both
normal and catastrophic times.
I have devoted the last ten years of my professional life in paleontology to constructing an unorthodox theory for explaining the lack of expected patterns during normal times—the theory of punctuated equilibrium. Niles Eldredge and I, the perpetrators of this particularly uneuphonious name, argue that the pattern of normal times is not a tale of continuous adaptive improvement within lineages. Rather, species form rapidly in geological perspective (thousands of years) and tend to remain highly stable for millions of years thereafter. Evolutionary success must be assessed among species themselves, not at the traditional Darwinian level of struggling organisms within populations. The reasons that species succeed are many and varied—high rates of speciation and strong resistance to extinction, for example—and often involve no reference to traditional expectations for improvement in morphological design. If punctuated equilibrium dominates the pattern of normal times, then we have come a long way toward understanding the curiously fluctuating directions of life’s history. Until recently, I suspected that punctuated equilibrium might resolve the dilemma of progress all by itself.
I now realize that the fluctuating pattern must be constructed by a complex and fascinating interaction of two distinct tiers of explanation—punctuated equilibrium for normal times, and the different effects produced by separate processes of mass extinction. Whatever accumulates by punctuated equilibrium (or by other processes) in normal times can be broken up, dismantled, reset, and dispersed by mass extinction. If punctuated equilibrium upset traditional expectations (and did it ever!), mass extinction is far worse. Organisms cannot track or anticipate the environmental triggers of mass extinction. No matter how well they adapt to environmental ranges of normal times, they must take their chances in catastrophic moments. And if extinctions can demolish more than 90 percent of all species, then we must be losing groups forever by pure bad luck among a few clinging survivors designed for another world.
Heretofore, we have thrown up our hands in frustration at the lack of expected pattern in life’s history—or we have sought to impose a pattern that we hoped to find on a world that does not really acquiesce. Perhaps now we can navigate between a Scylla of despair and a Charybdis of comforting unreality. If we can develop a general theory of mass extinction, we may finally understand why life has thwarted our expectations—and we may even extract an unexpected kind of pattern from apparent chaos. The fast track of an extraordinary meeting in Indianapolis may be pointing the way.
Postscript
As a happy irony of science at its best, any essay on exciting new material guarantees its own swift oblivion as discovery augments. I almost eliminated this essay as superseded (as others, not lamented, have disappeared), but finally decided to keep it without change as an honest expression of immediate excitement written while all the new ideas still rang in my ears. Thus, I have not tried to revise (and change the tone) with published versions that have appeared since the original verbal presentations. The essays of section 8 update the second part on mass extinction, while Seilacher’s bibliographic reference may be consulted for more information on the first part.
I cannot, however, resist one update in pictorial form. In December, 1984, Dolf Seilacher sent me the following copy of his first attempt to draw the entire Ediacaran fauna in the light of his new theory. No theme is more basic to this book, and to its convictions about the centrality of history, than the importance of taxonomy viewed, not as a neutral hatrack for the objective facts of nature, but as a theory that constrains and directs our thinking. Seilacher’s figure stunned me with the particular joy of seeing something entirely new in familiar objects. All my professional life, I have viewed the Ediacaran organisms as ancestors of later, modern phyla. I have so classified them in my mind.
Spriggina
(row 1) went with the worms,
Charnia
(row 1) with the corals,
Cyclomedusa
(row 3) with the jellyfish, and
Tribrachidium
(row 3) with the echinoderms. Placed in these disparate pigeonholes, I simply never saw the similarities that now jump out at me (although, in some “objective” sense, the similarities were always “there”). Now I can see Seilacher’s point so clearly—a community of quilted, sheet-like structures with different axes of growth and symmetry. Taxonomy is a dynamic and creative science of history.
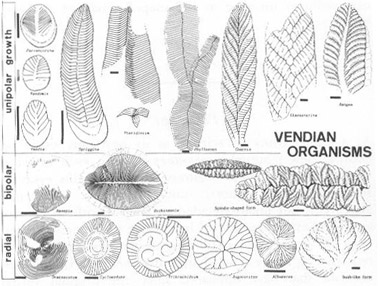
Seilacher’s first drawing of the new taxonomy for Ediacaran organisms. Note how, arranged this way according to different styles and axes of growth—rather than by presumed relationship to later organisms—we can easily see the common features and coordinating themes of all Ediacaran organisms.
ON OCTOBER
1, 1939, a month after Stalin and Hitler signed their nonaggression pact, Winston Churchill described Russian policy as “a riddle wrapped in a mystery inside an enigma.” All professions have their classical enigmas, although they can rarely boast a Churchill to describe them so well. My own field of invertebrate paleontology has a formal Latin designation for its mysteries. They are gathered into a wastebasket category of classification called Problematica—animals of unknown zoological affinity, even though their fossils may be both well preserved and abundant. The resolution of a problematic group becomes a cause for general rejoicing among paleontologists. Early in 1983, the most resolute of all paleontological mysteries finally yielded at least halfway. I wish to recount this tale and to explain why it has a general importance far transcending the simple delight of discovery.
The most vexatious of all fossil Problematica had been the conodonts (see photo on chapter 16). As their name (“cone tooth”) implies, conodonts are tiny, tooth-shaped structures of phosphatic composition. (Most hard parts of marine invertebrates are made of calcium carbonate, although some, including conodonts, are calcium phosphate. Vertebrate bone is also phosphatic, leading many paleontologists to speculate that conodonts might be the teeth of extinct fishes.) Conodonts range in size from microscopic dimensions to about 3 mm in maximum length, and in age from Cambrian to Triassic—about 580 to 200 million years ago.
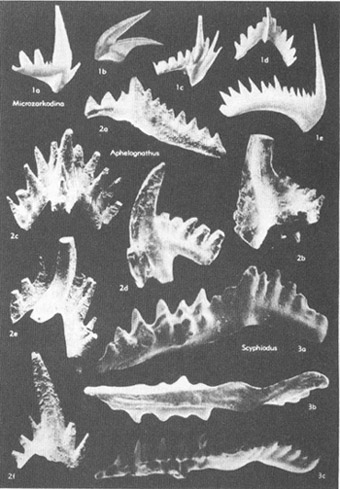
A selection of conodonts, tooth-like fossils of great stratigraphic value.
REPRINTED FROM NATURAL HISTORY
.
Many Problematica are rare and insignificant creatures. Conodonts, on the other hand (and despite their diminutive size), are among the most important of all fossils. They are found in abundance in a wide variety of rocks, and they evolved quickly, thus enhancing their value in correlating strata (since each short segment of time features its unique conodonts). Conodonts are therefore among the half dozen most important groups of fossils in the science of biostratigraphy—the dating and correlation of rocks by their fossil remains and still (despite growing interest in biological and evolutionary problems) the most important source of employment for paleontologists. One expert has stated that conodonts are “superb tools in worldwide biostratigraphy, and their value in Cambrian through Triassic rocks is not exceeded by any other group of fossils.” Imagine, then, our frustration: such practical importance and we don’t even know what kind of animal they represent. No other group of such importance lies among the Problematica.
Conodonts are evidently the only hard parts (and therefore the only portions generally preserved as fossils) of an otherwise soft-bodied creature. But what kind of animal, and how can you tell from a few separated toothlike structures? When conodonts were known only as isolated, disarticulated elements—the situation from their discovery in 1856 until 1934—we had almost no anchor for any sensible opinion, and speculation ran rampant. Conodonts were placed in almost every major group of the plant and animal kingdoms, from support structures for algae to copulatory organs of nematode worms. The most common opinions cast them as jaw elements either of annelid worms or of fishes.
In 1934, the first so-called assemblages of conodonts were discovered—articulated elements joined together in definite and invariant patterns. With their bilateral symmetry and gradation of toothlike elements from large to small, these assemblages suggested even more strongly that conodonts acted as food-gathering structures (either directly as teeth or indirectly as hard supports for fleshy or ciliary food collectors). More fanciful hypotheses of affinity faded away, and the idea that conodonts were jaw elements of some wormlike or fishlike creature gained further strength. But we still had no direct evidence for the conodont animal.
Then, in 1969, paleontologists throughout the continent gathered at the Field Museum of Natural History in Chicago for the first North American Paleontological Convention. (I well remember, as a wet-eared, first-year assistant professor, sitting there in awe amidst all the greats of my profession and thinking, “If the Russians—or the Chinese, or whoever—wanted to destroy this entire profession, one bomb….” And then concluding confidently: “But why would they bother?”) At the plenary session, a dramatic announcement was made—the conodont animal had finally been found. A soft-bodied creature from Montana had been discovered with conodonts inside, in a position interpreted as the mouth or anterior gut, where food might be chewed or macerated. These animals possessed other features that seemed to ally them with chordates, primitive members of our own phylum (including all vertebrates), and they were named conodontochordates.
It was, unfortunately, a false alarm. Further study revealed that the conodonts lay further back in the gut—in a position strongly implying that they had been swallowed by the beast. Moreover, their distribution was inconsistent with what we know about conodont assemblages. One conodontophore contained parts of two distinct assemblages, a clear indication that two conodont animals had somehow found their way within. Another contained conodonts varying too much in size for a reasonable inference that they came from the same organism. A third had no conodonts at all in the favored place. Clearly, the so-called conodontophores had been eating conodont animals and often retained conodonts of more than one individual in their gut. This news may have disappointed paleontologists, but it did not debase the significance of the discovery. The conodontophore is a conodont eater, not a conodont animal, but it remains an outstanding conundrum in its own right. Instead of resolving one of the Problematica, we had added another to our burgeoning list. So be it. The addition of one intriguing mystery is nearly as good (and often more interesting) as the solution to another.
Contrary to the romantic image of science and exploration, many important discoveries are made in museum drawers, not under adverse conditions in the parched Gobi or the freezing Antarctic. And so it must be, for the nineteenth century was the great age of collecting—and leading practitioners shoveled up material by the ton, dumped it in museum drawers, and never looked at it again. One of the great zoological discoveries of our century, the primitive segmented mollusk
Neopilina
, had been dredged from the deep sea, placed in a vial and labeled with the name of a limpet-shaped snail (for its external shell maintains such a shape)—where it remained for several years until H. Lemche turned the vial over to look at the soft parts and saw the segmented gills.
I am delighted to report that the conodont animal has now, and apparently truly this time, been found—in a museum drawer in Scotland. My friend Euan Clarkson was rummaging through some material of Carboniferous age (about 340 million years old) collected by D. Tait during the 1920s, when he noticed the impression of a worm-shaped creature with conodonts at the front end, right where the mouth should be. Since Clarkson is not an expert on conodonts, he called in some colleagues to verify and extend his discovery. Their results have just been published (Derek E.G. Briggs, Euan N.K. Clarkson, and Richard J. Aldridge, in bibliography).
Our fossil record is almost entirely the history of hard parts—bones, teeth, shells, and plates—because soft structures decay quickly and do not fossilize. Under very special circumstances, soft parts can be preserved, and these rare windows on the true diversity of past life are among the most precious of our fossil localities. For the 600 million years that multicellular animals have dominated our earth’s fauna, we have no more than a dozen or so extensive deposits of soft-bodied creatures. Most famous are the carbonized films of weird and wonderful creatures from the Burgess Shale, Cambrian of Alberta (some 550 million years old, and most ancient of our extensive windows); animals preserved within ironstone concretions from the Mazon Creek Formation of Illinois, Carboniferous period (350–270 million years old); and the Jurassic (180–130 million years) lithographic limestones of Solnhofen, Germany, where remains of
Archaeopteryx
, the first bird, feathers and all, were discovered.
The conodont animal comes from one of our smaller windows, the so-called shrimp band within the Granton Sandstones found east of Edinburgh. The Granton Sandstones are a sequence of lake and lagoonal sediments deposited in fresh or slightly saline water. This basin was occasionally flooded by the sea, and the shrimp band represents one such marine incursion. Its soft-bodied fauna was preserved because two unusual conditions prevailed during this brief flood. First, the bottom waters apparently lacked oxygen. No animal scavengers or bacteria could live on the lake floor, and dead animals floating down from above were not dismembered or decomposed. (We make these inferences because the shrimp band displays continuous, fine-layered sedimentation, an indication that no creatures burrowed or plowed through the bottom muck.) Second, the basin was stagnant and virtually devoid of currents. Thus, fragile, soft-bodied creatures were not pulled apart but floated gently down to be buried intact.
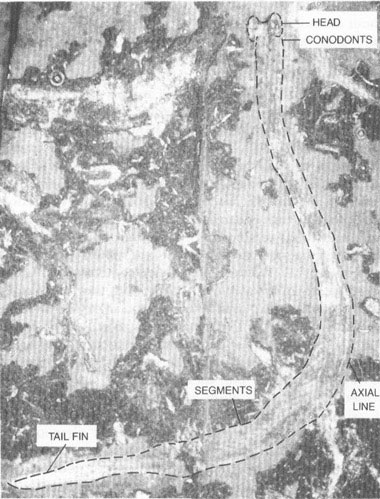
Fossil of a conodont animal (here outlined with dashes) recently found in a museum drawer in Scotland. Its taxonomic position is controversial; it may best be placed in a new phylum of its own.
REPRINTED FROM NATURAL HISTORY
.
The conodont animal is wormlike in appearance, some 40.5 mm long, but no more than 2 mm wide (see photo on chapter 16). Its head end seems to be cleft, with two broad lobes surrounding a central depression (entrance to the mouth, perhaps). Just behind the head, conodonts are affixed along one edge in a sensible position for the mouth. They occur in three groups and contain elements of a well-known assemblage. Thus, Clarkson and his colleagues did not need to invent a name for their creature; they included it within the genus
Clydagnathus
, first established in 1969 for the skeletonized conodonts alone. A few faint lines run along the interior of the animal, parallel to its sides. Whether these represent a gut, a nerve tube, perhaps even the chordate notochord, we do not know. About two-thirds of the way back, and extending nearly to the posterior end, we find an intriguing sequence of repeated segments, some thirty-three in all, sloping at an angle to the midline of the body. Finally, one edge of the posterior end seems to sport a sequence of projections, interpreted as fin rays. Not much else worthy of mention has been preserved. At least the structures of
Clydagnathus
confirm one old assumption about conodont elements—they represent the only hard parts of an otherwise entirely soft-bodied creature. No wonder we had so little previous success in determining their affinity.
As I said at the outset, Clarkson and his colleagues have solved only half the conodont problem. They have found the elusive animal, but they do not know where it belongs. Of modern animal phyla, only two seem worthy of discussion as a possible taxonomic home for the conodont animal. Perhaps it is a chordate—that is, a prevertebrate member of our own phylum. Yet, each potential similarity with chordates scarcely carries conviction. The slender and flattened eel-shaped body reminds us of some chordates, but we find the same general shape in several other phyla as well. The faint lines parallel to the animal’s side could represent such chordate structures as the notochord, but they may simply be remnants of the gut as well, an organ shared by virtually all “higher” animals. The apparent fin rays of the posterior end suggest chordate affinities, but similar structures occur in several other phyla as well. The V-shaped segments seem to say “chordate,” but these structures are so poorly preserved that we cannot really distinguish between a chordate style of segmentation and the patterns of several other phyla with serially repeated elements. In short, we find a few general and superficial similarities with chordates but nothing specific, and surely nothing that would warrant any firm, or even tentative, placement within our phylum.
The Chaetognatha, or arrow worms, a small marine group located not far from chordates on our evolutionary tree, include the only other viable candidates for a link between the conodont animal and some modern group. Chaetognaths are armed with grasping spines that flank the mouth in two lateral sets. These spines bear a superficial resemblance to conodonts, but they are made of chitin, not calcium phosphate. Chaetognaths also have tail fins not unlike those of the conodont animal. But they also have lateral fins, and such structures are not present on the conodont animal (in an area of the body—the posterior—where preservation is detailed and excellent). In short, chaetognaths seem an even less worthy prospect than chordates as a home for the conodont animal.
