The Cerebellum: Brain for an Implicit Self (5 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

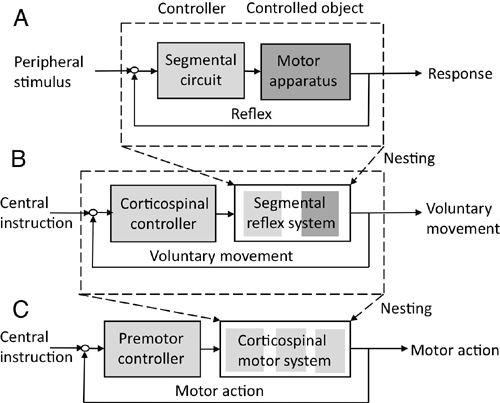
Figure 12. The two-step nesting of a reflex within the premotor cortical control of a motor action.
(A) and (B) are similar to those in
Figure 11
. (C) shows a further nesting for control at a cortical level.
Finally, the imitation hypothesis discussed in
Section 6
provides the eighth strategy used by the CNS to evolve voluntary motor control systems utilizing the reflex control systems formed in the brain stem and spinal cord as a basement structure.
In the study of voluntary movements, the convention is to begin with analysis of a simple movement such as flexion of an elbow. However, voluntary movements that we perform daily are parts of “motor actions” that involve the participation of many body parts and even use of a tool to attain a purposeful goal. Moreover, motor actions also involve perceptual and conceptual activities, for example, in piano playing and dancing. It has been suggested that an “action schema” representing and coding motor actions is expressed in the posterior parietal and premotor (Broadmann’s area 6, see
Figure 2
) cortices (
Jeannerod, 1994
). For the present purposes, an action schema can be considered to be a cerebral cortical model.
In primates, the premotor cortex expands rostrally from the primary motor cortex. It is generally assumed that the premotor cortex, in particular its dorsal part, plays a major role in computing and controlling complex motor actions (
Wise et al., 1997
). Moreover, the premotor cortex includes “mirror neurons,” which discharge similarly during a motor action performed by the self or by another
individual, as discovered by Rizzolatti and his colleagues (
Rizzolatti and Craighero, 2004
). Based on these and other lines of evidence presented in
Chapter 16
, we assume that the premotor cortex acts as the controller of motor actions. The premotor controllers act on controlled objects, which nest the primary motor cortex and lower motor systems (see
Figure 12C
). The postulated action schema is assumed to reside in the temporoparietal cortex and provide a cerebral cortical model to the premotor cortex. The same control system structure may apply to tool use if a tool is represented in an action schema together with body parts (
Chapter 15
).
Action schema may include two related concepts (actually CNS properties) that are prominent in psychology: the “body schema,” which possesses a continually updating map of the self’s body shape and postures; and the “motor schema,” the self’s long-term memory structure capable of being retrieved as a whole, and then controlling the elaboration of motor skills composed of complex actions and movements. Both schemata are acquired or at least further refined by learning (Arbib, 2005;
Stamenov, 2005
). Along with the model-based control concepts discussed above (
Section 5
), these body/motor schemata can be considered as components of cerebral cortical models representing the forward and inverse models of the controlled object (
Figure 8
). These cerebral cortical models are presumably acquired during the initial learning of motor actions. As learning advances, the acquired body schema and motor schemata are transferred to cerebellar internal models (
Chapter 15
).
The various forms of control systems discussed above operate in the physical domain. Our CNS moves various body parts daily by contracting or relaxing muscles to make a purposeful movement. Analogously, we manipulate daily a thought in the mental domain. For example, we use languages, make evaluations, and come to decisions, these being major components of human intelligence. Formalistically, as a controlled object, an idea or concept in the mental domain is analogous to a body limb in the physical domain. At present, however, there is as yet no experimental or computational way to bridge the physical and mental domains that operate in the CNS. Hence, any postulated thought control mechanism has no unequivocal representation in neuroscience.
Nevertheless, we find some concepts in the field of psychology that mediate physical and mental domains. For example, there is the term “mental model,” which Craik (
1943
) and Johnson-Laird (
1983
) defined as a psychological substrate for a mental representation of real or imaginary situations. It is defined as a small-scale model of reality that the mind constructs and uses to reason, underlie
explanations, and anticipate a future event. More concretely, mental models in psychology are representations of images, concepts, and ideas. One may also recall another term in psychology called “schema,” which Jean Piaget (1896–1980) defined as being formed in a growing child learning to interpret and understand the surrounding world (
Piaget, 1951
). Piaget’s schema includes both a category of knowledge and the process of obtaining that knowledge.
Currently, the above two concepts are not mechanistic, and they lack a computational basis. Hence, the examples presented below in
Chapter 17
, “Cognitive Functions,” are largely hypothetical. Nevertheless, I believe that once neural correlates of a mental model and Piaget’s schema have been established, the present well-accepted control system models that exist in the physical domain will be shown to also apply to the mental domain, and thereby help understand various cognitive mechanisms.
Figure 8
, which shows model-based control system designs, may be referred to for considering a mental model as a controlled object. For the present, computer simulation cannot reproduce this model because it lacks a computational basis. This difficulty is like the one that arose in the field of artificial intelligence. More than 50 years ago, a group of computer scientists proposed a study that would “... proceed on the basis of the conjecture that every aspect of learning or any other feature of intelligence can in principle be so precisely described that a machine can be made to simulate it.” These scientists were eager to make “... an attempt to find how to make computers that use language, form abstractions and concepts, solve kinds of problems now reserved for humans, and improve themselves” (
McCarthy et al., 1955
). This tempting approach in artificial intelligence, however, remains unsuccessful because it lacks the clarification provided by neural network mechanisms that can encode a concept or a specific piece of knowledge.
Another profound question is how the operation of a neuronal circuit can be undertaken with conscious awareness. Sigmund Freud (1856–1939) and many more recent researchers have emphasized that only a few of the activities of the CNS are executed consciously. For example, one cannot bring to conscious awareness the thought processes involved in improving motor skills (e.g., skiing) by training (non-declarative memory). In contrast, one can readily recall cognitive experiences (declarative memory) (
Squire, 2009
). In other words, the neuronal circuits implicated in non-declarative memory are remote from the mechanisms of conscious awareness, whereas those involved in declarative memory are closely connected to conscious awareness. On the other hand, it has been shown that electric or transcranial magnetic stimulation (TMS) of the neocortex usually evokes vivid sensations or perceptions (
Penfield and Perot, 1963
; Coway and Welsh 2001), whereas stimulation of the cerebellum (
Riklan et al., 1976
;
Koch et al., 2006
) and basal ganglia (
Chen et al., 2006
) has no impact on conscious awareness. Conventionally, intelligence has been considered to require consciously activated cortical functions, but a substantial part of it is probably exerted subcortically and consequently unconsciously. In fact, intuitive thought is an important part of intelligence, but it is exerted unconsciously without obvious reasoning (
Chapter 17
).
Neuroscience has reached a level of sophistication that is on the verge of addressing neural mechanisms underlying intelligence and conscious awareness. It seems likely that research on the cerebellum will be on the forefront of this endeavor.
In the chapters that follow, the neuronal circuits of the cerebellum are decomposed and reconstructed as explained in this chapter. Early and recent historical material is presented in
Chapters 2
and
3
, and
Chapters 4
–
9
update understanding of the cerebellum as an elaborate neuronal and molecular machine. Next, recent progress is presented about how this machine provides an advanced type of systems control for reflexes (
Chapters 10
–
12
) and voluntary movements (
Chapters 13
–
15
). The material covered in
Chapters 10
–
15
reviews findings that came mainly after my 1984 book,
The Cerebellum and Neural Control
, and Barlow’s 2002 monograph,
The Cerebellum and Adaptive Control
.
Chapters 16
and
17
examine the new possibility that the involvement of the cerebellum goes beyond movements to the higher-level functions of motor actions and cognition. The last
Chapter 18
, “
Concluding Thoughts
,” includes a summary of points made in preceding chapters about structural-functional relationships in neuronal circuit structures of the cerebellum as developed step by step in evolution.
The decomposition-reconstruction method provides a logical and effective approach to studying the structure-function relationships of neuronal circuits. They are composed of local multilayered networks that interconnect globally to form neural control systems. Reflexes are the most fundamental units of neuronal circuits. Multi-input, half-fused, hybridized, mutually interacting, compounding, neuromodulating, nesting, and imitating are the eight ways to integrate reflexes into complex movements, voluntary movements, and innate behavior. A further integrated control is needed for both motor actions and, as yet to be determined, the mechanisms of cognitive thought.
The cerebellum is a regular part of the CNS in vertebrate animals. It is recognized in lampreys, fish, amphibians, reptiles, birds, and mammals, up to humans. Among nonvertebrate animals, a cerebellum-like structure has been reported in octopus ganglia (
Hochner et al., 2006
), but its presence in other nonvertebrate species is unclear. The unique morphology of the cerebellum has been studied thoroughly for over a century. This led to the establishment of a map commonly applicable to various animals. Characteristically, the map initially involved the cerebellum’s connections with the vestibular nuclei in the medulla oblongata and with the spinal cord. The map then expanded in parallel with the further evolutionary development of the cerebral cortex. Distinctive involvement of the cerebellum in the acquisition of motor skills has also been uncovered on the basis of a large amount of data accumulated from lesion studies on animals and clinical investigations in humans. Furthermore, the cerebellum has been examined extensively by microscopy, revealing the presence of Purkinje cells and other cells of unique morphology. In this chapter, we trace the history of how these traditional views of the cerebellum have been formulated.
Erasistratus (304–250 BC) of Greece distinguished the cerebrum from the cerebellum (
Malomo et al., 2006
). Anatomical descriptions of the brain were almost complete during the Renaissance period, as we see in fine sketches of human brains drawn at that time. In the middle of the twentieth century, the characteristic morphology of the cerebellum was rigorously analyzed, and the data were compiled in two monumental volumes (
Jansen and Brodal, 1954
;
Larsell, 1970
).
When viewed from above, the mammalian cerebellum appears like a butterfly (Color Plate I). The middle part lying along the anterior-posterior axis is called the vermis because of its wormlike appearance. From the vermis, the hemispheres expand to the right and left like wings. Deep grooves divide the wings into three parts; the primary fissure separates the anterior and posterior lobes, and the posterolateral fissure separates the flocculonodular lobe from the posterior lobe. These divisions are further subdivided by transversely running grooves into individually named lobules (Color Plate II). Each lobule contains shallow folds, i.e., folia. The cerebellum is subject to a greater range of species variations than any other parts of the brain, but after much effort for over a century, anatomists reached a fundamental design of the cerebellum common to various vertebrate species (
Larsell, 1970
). It consists of ten divisions (lobules I–X) anteroposteriorly laid along the vermis and their transverse expansion into the hemispheres (lobules HI–HX). These divisions are shown schematically in an unfolded surface of the cerebellum (Color Plate II).
