The Cerebellum: Brain for an Implicit Self (37 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

The three-staged learning mechanism proposed previously for motor actions may explain a common observation in patients afflicted with Alzheimer’s disease (AD). They exhibit serious impairments of memory and intellect, yet they retain
nurtured patterns of daily life such as eating, dressing, and bathing. This suggests that in AD patients, whereas body/motor schemas in the cerebral cortex are impaired, internal models in the cerebellum are preserved. Indeed, AD patients are characterized by the regional impairment of cerebral glucose metabolism in
neocortical association areas, including the posterior cingulate, temporoparietal, and frontal association cortices, whereas the primary visual and sensorimotor cortices, basal ganglia, and cerebellum are relatively well preserved (
Herholz, 2003
).
From a modelistic viewpoint, it would be difficult for a single controller to skillfully perform a complex motor action with the aid of but one internal model. Probably, multiple controllers and associated internal models are involved simultaneously, and there is a mechanism for selecting an appropriate combination for a specific condition (
Wolpert et al., 2003
). The MOSAIC model, using combinations of forward- and inverse-model-based controls, can simulate such mechanisms (
Chapter 15
,
Section 6
).
The premotor cortex of monkeys contains “mirror neurons.” Their discharge patterns are quite similar when a particular object-directed action (such as grasping, tearing, manipulating, holding, and bringing an object to the mouth) is taken and when the same action is observed while being undertaken by another individual (another monkey or a human experimenter) (
Rizzolatti and Craighero, 2004
). Correspondingly, in an fMRI study on human subjects, motor actions performed using different effectors (mouth, hand, foot) induced a somatotopically organized activation of the premotor cortex (
Buccino et al., 2001
). Mirror neurons are located in the ventral premotor cortex (area F5; Color PlateXVI) of the monkey, where many other cells are also located that code for visually guided actions rather than observed actions. Mirror neurons do not respond to the object alone, but they discharge during a reach to an object placed out of sight, as long as the intention of the reach and grasp action is clear. Thus, these neurons are not driven simply by visual input, but rather, they respond to an object-directed action.
Cerebral cortical areas associated with mirror neurons have been mapped in monkeys and humans, and neurons responding similarly to F5 mirror neurons were found in area PF of the posterior parietal cortex (
Iacoboni et al., 1999
) (Color Plate XVI). They coded more specifically for the kinesthetic and somatosensory components of an action. Another relevant area has been located in the superior temporal sulcus (
Iacoboni et al., 2001
;
Carr et al., 2003
). It was activated during both hand action observation and imitation even in the absence of direct vision of the imitator’s hand. Motor-related activity was greater during imitation than during control motor tasks. The observed actions and the copies of these actions as made by the imitator may interact in this area (
Iacoboni et al., 2001
).
Many conceptual models have been proposed to reproduce the possible functions of mirror neurons, in particular, the ability to imitate an object-directed action (
Miall, 2003
;
Oztop et al., 2006
;
Iriki, 2006
). Here, we consider a simple plausible control system design for this function. Assume that F5 mirror neurons operate as part of the premotor cortical action controller for the controlled object nesting a corticospinal and a segmental motor system. Assume also that the PF area represents kinesthetic and somatosensory components of an action (see previous discussion) as a body schema. Assume further that the premotor cortex receives feedback information about self-generated actions via a somesthetic pathway, and that it also receives visual information about an action performed by another individual. The most crucial assumption is then that the preceding two pathways leading to F5 mirror neurons are commonly mediated by the superior temporal sulcus region (
Figure 52
).
Figure 52. A possible control system structure for mirror neurons.
Mirror neurons are assumed to be a component of premotor cortical neurons, which serve as a controller of motor actions. To behave as mirror neurons, they should be equipped with a comparator of the self-generated and observed individual’s movements, which is likely located in the superior temporal sulcus (STS). Other abbreviations: PF, parietal cortex defined in Color Plate XVI; t, broken arrow indicating close connections between PF and STS.
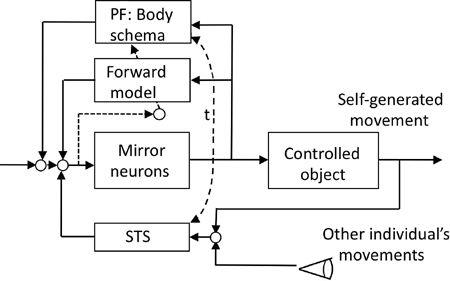
In this postulated system, a group of F5 mirror neurons serves as an action controller, whereas PF mirror neurons help these F5 mirror neurons control skillfully an action by providing a body schema. During repeated trials of a motor action, a cerebellar forward model copies the body schema from the PF region so
that the skillful motor action can be performed more automatically. F5 mirror neurons are driven to discharge by instruction signals from a higher center, and they are also driven by feedback from self-generated actions via the superior temporal sulcus area. This area also receives information about the observed “other-individual’s” motor actions (
Figure 52
). The superior temporal sulcus area is anatomically interconnected with the PF area, and together, they may share the body schema for generating information about performed actions. Of essential importance is the postulated neuronal mechanism of the superior temporal sulcus area, where information about an observed action replaces that of a performed action, provided the former imitates the latter.
The major role of mirror neurons is generally considered to be in recognizing the actions of others (action recognition). Recently, however, a human fMRI study showed that premotor mirror neurons exhibited greater activity when an action was observed in the presence of a specific context, such as the background scenery. This suggested that such neurons were interpreting the intention of the observed action (intention understanding) (
Iacobini et al., 2005
). Mirror neurons have also been considered to be responsible for imitation learning—that is, learning to perform an action by seeing it done. However, because monkeys rarely learn by imitation, it does not seem to be a primary function of mirror neurons, which are commonly located in the cerebral cortex of humans and monkeys (
Rizzolatti and Craigher, 2004
). Nevertheless, fMRI activity observed in both humans and monkeys during their responding to an observed action was greater than that during responding to a non-movement-relative scene. The greater imitation-activated activity in the cerebral cortex was observed in the superior temporal sulcus area (
Iacoboni et al., 1999
,
2001
). In summary, the data to this point suggest that the imitation capacity involves use of the mirror neuron system.
The idea of sensory cancellation (
Chapter 15
) also applies to problems of motor actions. Schizophrenic patients often exhibit the delusion of alien control, which makes them misattribute self-generated motor actions to externally generated motor actions by others. Blakemore et al. (
2003
) explained this phenomenon as arising from the inefficient or erroneous operation of a forward model. That is, if an internal feedback through a forward model does not cancel the sensations induced by self-generated actions, then the latter will reach the level of consciousness in a fashion similar to that induced by external stimuli generated by others. In
this situation, the patient cannot distinguish self-generated actions from those generated externally by others. The alien control seen in subjects under hypnosis may likewise be explained as arising from abnormal operation of a forward model.
A brain imaging study revealed that the active movement attributed to another individual resulted in significantly higher activations in both the parietal cortex and the cerebellum than an identical movement correctly attributed to the self (
Blakemore et al., 2003
). Blakemore and Sirigu (
2003
) interpreted these observations as indicating that both the parietal cortex and the cerebellum provide internal models of a different nature. They speculated that whereas a cerebellar internal model makes rapid predictions about the sensory consequences of a self-generated movement commanded and controlled automatically at a low level of the brain, a model in the parietal cortex addresses more cognitive aspects of the same movement and this processing reaches the level of conscious awareness. This view is close to the previously postulated learning of motor actions except that here we assume that the parietal cortex first forms a series of body schemata, which are then copied by the cerebellum.
Another phenomenon suggesting the involvement of the parietal cortex in motor action control is the sensation of a phantom limb—that is, an amputated limb being still present and sensed on occasion to be in motion (
Frith et al., 2000
). This sensation is represented in the parietal cortex (
Ramachandran and Hirsten, 1998
), and it may arise from motor schemata for the limb that are retained after loss of the limb. An internal model in the cerebellum may also be retained, but it does not contribute to the phantom limb sensation because cerebellar events do not reach self-awareness.
Various types of sport and dancing demonstrate the ability of some humans to achieve superb motor control. In one type of motor action, the controlled objects are mainly body parts (e.g., in swimming, running, gymnastics). In another type, various tools are used (e.g., balls, bat, gloves). Throughout training, tools become incorporated into the motor control system as if they are newly added parts of the body. Such extended motor capability requires changes in the neural representation of the body schema, that is, updated maps of body shape and posture incorporated into the cerebral cortex (
Maravita and Iriki, 2004
). Lesion studies in right-handed patients have revealed that the left cerebral hemisphere is specialized for representing tool-use skills, but there is evidence that the left cerebral hemisphere is also specialized for representing these skills in left-handed individuals (
Johnson-Frey, 2004
). Together, these findings indicate that behaviors associated with complex tool use arise from functionally specialized networks involving temporal, parietal, and frontal areas within the left cerebral hemisphere. I believe that the resultant body schema will then be copied by an internal model in the cerebellum (
Chapter 15
). Semantic knowledge about familiar tools and their uses is stored also in the cerebral hemisphere, but it is uncertain whether or not this cognitive memory is relevant to the cerebellum.
Neural events underlying tool use have been studied in monkeys and humans. In one experiment, a monkey was trained to manipulate a stick to shift a cursor on a screen from a starting box to a target box. When the target box was suddenly repositioned, the monkey had to modify the ongoing movement to place the cursor within an also repositioned target box. This induced a significant increase in the complex-spike discharges of Purkinje cells located in the ipsilateral hemisphere and intermediate zone of the cerebellum. This finding was interpreted to mean that climbing fiber signals occurred when the motor state changed and/or when errors were occurring in the motor performance (
Wang et al., 1987
). In another study, monkeys performed a multijoint arm-reaching task, in which movement direction and distance were varied systematically. The complex-spike activity of relevant Purkinje cells was often correlated significantly with movement distance, usually in one direction. It was therefore suggested that the complex-spike discharge of Purkinje cells was spatially tuned and related strongly to the movement’s kinematics (Fu et al., 1997b).
In a study on humans, the subject manipulated a computer mouse to follow a moving small square target with a small cross-hair cursor on a screen. During continuous following, the position of the cursor was suddenly shifted by rotating it 120° around the center of the screen to provide a novel mouse condition. In the first training session, large regions of the cerebellum were activated significantly, but the extent of activation decreased during repeated test trials in parallel with a reduction in the rate of tracking errors. Eventually, certain subregions (near the posterior superior fissure) continued to be activated. This remaining activity may have represented an internal model being formed during the repeated test trials. This would imply a novel relationship between the movements of the cursor and the mouse. Because the subject could switch quickly between the old and the novel mouse, it seemed likely that there must be a neural mechanism for selecting an appropriate model out of many internal models of the various mouse situations (
Imamizu et al., 2000
).
