Life's Ratchet: How Molecular Machines Extract Order from Chaos (34 page)
Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann


FIGURE 7.9.
An example for the family tree of a molecular motor: the family of myosins. Reproduced/adapted with permission from Tony Hodge, M. Jamie, and T. V. Cope, “A Myosin Family Tree,”
Journal of Cell Science
113 (2000): 3353–3354.
A Myosin Family Tree
Tony Hodge and Jamie Cope
Abbreviations | |
| | |
Ac | Acanthamoeba castellanii |
Acl | Acetabularia cliftonii |
Ai | Aequipecten irradians |
At | Arabidopsis thaliana |
Bm | Brugia malayi |
Bt | Bos taurus |
Cc | Chara corallina |
Ce | Caenorhabditis elegans |
Cr | Chlamydomonas reinhardtii |
Dd | Dictyostelium discoidium |
Dm | Drosophila melanogaster |
En | Emiricella nidulans (Aspergillus) |
Eh | Entamoeba histolytica |
Gg | Gallus gallus |
Ha | Helianthus annus |
Hs | Homo sapiens |
Lp | Limulus polyphemus |
Ma | Mesocricetus auratus |
Mm | Mus musculus |
Oc | Oryctolagus cuniculus |
Ov | Onchocerca volvulus |
Pf | Plasmodium falciparum |
Pg | Pyricularia grisea |
Rc | Rana catesbeiana |
Rn | Rattus norvegicus |
Sc | Saccharomyces cerevisiae |
Sm | Schistosoma mansoni |
Ss | Sus scrofa domestica |
Tg | Toxoplasma gondii |
Tt | Tetrahymena thermophila |
Xl | Xenopus laevis |
Zm | Zea mays |
| | |
Adren | Bovine Adrenal (myosin I) |
Bb | Brush Border Myosin I |
CaA | Cardiac alpha (myosin II) |
CaB | Cardiac beta (myosin II) |
csm | Chitin synthase-myosin |
FSk | Fast Skeletal (myosin II) = striated |
FSkE | Embryonic Fast Skeletal (myosin II) |
HMWMI | High Molecular Weight Myosin I |
neur | Neuronal (myosin II) |
nm | Non-muscle (myosin II) |
PDZ | Human myosin with a PDZ domain. |
Peri | Perinatal (myosin II) |
sm | Smooth muscle (myosin II) |
Myosin VI is a motor protein like myosin V, but it moves in the opposite direction of myosin V. While myosin V moves lipid-encased cargo to the outside of the cell, myosin VI moves cargo to the inside. It is involved in
endocytosis
, which is the process through which the cell ingests molecules from the outside of the membrane. Myosin VII seems to be involved in the cells in our ears: Mutations in the myosin VII gene can lead to deafness. Myosin IX may play a role in sending signals to the cell that initiates rearrangement of its actin filament network. Clearly, considering the large number of motor proteins and the multiple functions they fulfill, more research will be needed to figure out what they all do.
Through hydrolysis, molecular motors turn ATP into ADP. Then they release ADP and the spent molecular fuel pellet floats away. What happens to it? Wherever possible, cells are keen on recycling. Why not recharge the ADP by reattaching a phosphate and turning it back into ATP? Indeed, this is what the cell does, and it happens in one of the cell’s most important organelles: mitochondria.
Mitochondria are the fuel recharging stations of cells (they are sometimes called the cell’s power stations). They use energy derived from food to recharge ADP. The details took decades to decipher; the process is surprisingly complicated and proceeds through many steps. Fortunately for our story, at the end of the process, there is a marvelous molecular machine—a machine that, like no other, illustrates how, at the nanoscale, different types of energy can be converted into each other with very high efficiency.
But before we talk about this amazing machine, let’s briefly look at how food turns into ATP-energy. As described in
Chapter 1
, scientists slowly came to the realization that the heat of the body was generated by
a slow burning process. Experiments by Lavoisier, Liebig, Helmholtz, and others showed that the energy generated from food is roughly equivalent to the burning of food in air. But how do our bodies burn food? In our cells, a number of complicated, multistep biochemical reactions can turn various food molecules—sugars, proteins, fat—into standard, high-energy molecules used by the cell. In each biochemical step, some energy is removed in the form of ATP or other energy-carrying molecules, and the end product has correspondingly less energy than the original food molecules. Thus, breaking down the burning process into many steps makes burning a very slow and, consequently, very efficient process.
In one such burning process, glucose (a sugar) is turned into two molecules of pyruvate (a small organic molecule), while generating two ATP molecules. Prior to the availability of free oxygen on our planet (courtesy of photosynthesis), this reaction was the end of the road. You could get two ATP molecules out of one glucose molecule, and that was it. In an aerobic bacteria (bacteria that do not use oxygen), pyruvate is waste. But pyruvate still has energy to offer. In aerobic cells (like our own), pyruvate becomes feedstock for the next set of biochemical reactions. All in all, the two pyruvate molecules that result from the burning of one glucose molecule generate another thirty ATP molecules. Now, that’s efficiency!
The first step in digesting pyruvate is to break it down further, releasing CO
2
(which we breathe out) and creating another intermediate product, an acetyl, which gets attached to a carrier called coenzyme A. This product, acetyl coenzyme A, then enters the next cycle, called the Krebs cycle after its discoverer, and after more CO
2
is released, we are left with several energy-carrying molecules called NADH and FADH
2.
I’ll spare you what these acronyms stand for—what’s important is that NADH and FADH
2
are electron-rich molecules, which means they can easily give up electrons.
When NADH gives up electrons to an enzyme embedded in the membrane of the mitochondrion, NADH turns into NAD
+
(
Figure 7.10
). The split-off hydrogen ion (H
+
, the same thing as a hydrogen nucleus, which in hydrogen’s case, is a single proton) passes through the membrane-spanning enzyme to the other side of the membrane. The electron is passed through several carriers along a chain of more enzymes (called complexes I, III, and IV), which use the electron energy to pump more protons across the membrane. In the end, this
electron transfer chain
succeeds
in pumping a lot of positively charged protons from one side of the membrane to the other. This movement of charges (protons) leads to the development of a
voltage
across the membrane. Thus, the purpose of the electron transfer chain is to recharge a biological battery. The first energy conversion is complete—the cell has turned chemical energy into electrical energy.
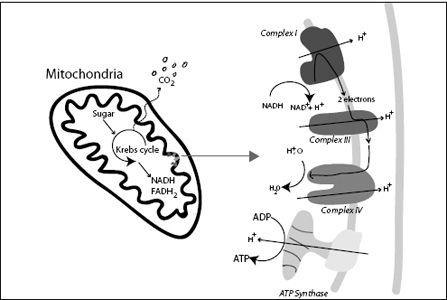
FIGURE 7.10.
Chemical energy in the form of sugar is converted in mitochondria into energy in the form of ATP. This process takes many steps. First the energy is fixed in molecules called NADH and FADH
2
. These molecules are then processed by different molecular machines in the mitochondrial membrane; the machines use the energy to pump hydrogen ions (H
+
) from one side of the membrane to the other. NADH is processed by complex I, while FADH
2
is processed by complex II (not shown). The excess of hydrogen ions on one side of the membrane is then used to drive the ATP synthase, which recharges ADP to ATP.
