Life's Ratchet: How Molecular Machines Extract Order from Chaos (35 page)
Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann

The idea of storing energy as an excess of protons on one side of a membrane goes back to the Scottish biochemist Peter Mitchell, who in 1961 suggested this “chemi-osmotic” process as a key to understanding mitochondria. His ideas were subjected to much criticism. Most biochemists believed in a purely chemical process of recharging ADP. Many years were wasted searching for an enzyme that would chemically recharge ADP, but none was found. Finally, Mitchell’s ideas were accepted and he was
awarded the 1978 Nobel Prize for Chemistry. In the late 1970s, however, the details of how it all worked were still quite sketchy. How did the stored electrical energy lead to the recharging of ADP molecules?
ATP SYNTHASE AND THE AMAZING SPINNING BATON
Wayne State University hosts the Ahmed Zewail Gold Medal Award and lecture (named after the Nobel Prize–winning Egyptian scientist who pioneered ultrafast measurements of chemical reactions). In 2010, the recipient of the award was another Nobel laureate, Sir John E. Walker, who deciphered the structure of ATP synthase, the molecular machine that constitutes the last step in the slow-burning process in our bodies. I thoroughly enjoyed Walker’s lecture, and during the question and answers, I asked about what was known about the evolution of these amazing machines. He raised an eyebrow and responded, in the laconic style of English intellectuals, “I usually only get questions like this in Texas,” alluding to the creationists’ use of the intricacies of our cells as proof of special creation. I laughed and assured him that I was genuinely interested in evolution and was not from Texas (my German accent should have been a giveaway).
Walker shared his Nobel Prize with UCLA biochemist Paul Boyer. Where Walker figured out the structure of ATP synthase, Boyer figured out how it worked. ATP synthase, as the name suggests, is a machine that makes ATP. The enzyme does not make it from scratch, but rather recycles ADP by attaching fresh phosphate groups. Attaching a phosphate to ADP and turning it into ATP requires energy, and ATP synthase takes this energy from the stored electrical energy provided by the electron transfer chain: The protons, laboriously moved to the outside of the mitochondrial membrane by the complexes of the transfer chain, are now pumped back—but not before giving up their electrical energy to the synthase machine.
In 1977, Boyer suggested that the flow of protons back through the membrane would drive a little rotary motor. Like the dial on a gumball machine, each turn of the motor would perform a different step of the ATP synthesis: binding an ADP and a phosphate, attaching the phosphate, releasing ATP. All of these steps would happen in the same enzymatic
pocket. The rotation would somehow modify this pocket during each turn so that it would be most suitable for the particular reaction step it was assisting. Having three such multifunction pockets would allow three ATPs to be produced per full turn of the machine.
Boyer also suggested that the energy supplied from the protons moving across the membrane (the proton-motive force, as it was called) was not used to attach a phosphate to an ADP. This apparently happened readily in the pocket of the enzyme. Instead, energy was needed to
release
the newly formed ATP from the pocket. As Boyer and his group continued their research, using a special isotope of oxygen,
18
O, to follow the movement of molecules, they discovered a curious phenomenon: When they ran the process in reverse, letting the synthase break down ATP, rather than synthesizing it, they found that removing the resulting ADP from the surrounding solution stopped the reaction. This made no sense. In chemical reactions, the removal of the product will only speed up a reaction, since it creates a large imbalance between reactant and products. How could the removal of the product, ADP, stop the reaction? One of Boyer’s students suggested that the still-unknown enzyme responsible for ATP synthesis (or breakdown, if run in reverse) only worked when somewhere in the enzyme, ADP was attached. This idea implied some kind of collaborative, allosteric interaction within the enzyme. Slowly, it became clear that the enzyme had three catalytic sites, which processed ADP in a sequential, coordinated way. The finding led Boyer to suggest that the ATP synthase went through a rotational cycle as it attached phosphates to ADP.
An interesting suggestion, but this theory only became widely accepted when Walker solved the structure of the F
1
part of the synthase. F
1
is the part Boyer identified with the dial—it performs the actual ATP synthesis. F
1
consisted of three identical units (each in turn consisting of two subunits, a and b) arranged in a circle like petals on a flower. Moreover, each identical b subunit had an ADP/ATP binding pocket. This circular arrangement was highly suggestive of rotary motion. But this motion was not proven until 1997, when a stunning experiment directly visualized the rotation of ATP synthase using fluorescence methods. Biochemist Hiroyuki Noji and his coworkers, then at Tokyo Institute of Technology, attached a short fluorescently labeled actin filament to the top of the F
1
unit of a single ATP synthase. When the researchers fed the synthase with
ATP, the machine ran in reverse, breaking down ATP into ADP and using the energy of ATP hydrolysis to fuel the rotation. This rotation then spun the attached actin filament around, like a majorette spinning a baton. Observing the actin filament in their microscope, Noji and colleagues saw that the F
1
unit rotated clockwise at a pretty good clip, up to four rotations per second. Moreover, the generated force was appreciable—by their estimates as much as 45 pN.
The efficiency with which the machine could turn energy from ATP hydrolysis into a rotation was astounding. In 2000, Noji’s group found that the machine was at least 88 percent efficient. Such a high efficiency is unheard of for macroscopic machines, but it gives us an idea of how the human body can be so efficient overall. We are based on tiny nanomachines—and only a nanomachine can be this efficient!
Noji’s experiment proved that the ATP synthase was a rotary machine, but the researchers had run the machine in the opposite direction of how it would be used in mitochondria. They had seen that a clockwise turn broke down ATP. It seemed obvious that a counterclockwise turn would achieve the opposite: reattaching phosphates to ADP. But this was just conjecture until experimentally proven. Unfortunately, it turned out to be difficult to observe ATP synthase in its usual environment in the mitochondria of a living cell. Once you remove the synthase from the cell, it is removed from the rest of converting machinery. Thus, the proton-motive force to drive the motor cannot be established and the motor cannot be driven in the direction that converts electrical into chemical energy.
Finally, in 2004, Noji’s team found a way. Instead of letting the protons do the work, they attached a magnetic bead to the center of the F
1
unit of the synthase. Then, using a rotating magnetic field, they forced the magnetic handle either clockwise or counterclockwise. In other words, they hand-cranked the motor. When they cranked it clockwise, the amount of ATP in the surrounding solution decreased. The F
0
unit acted as an ATPase, splitting off phosphates from ATP and releasing ADP. This confirmed their previous experiments. However, when they cranked the unit counterclockwise, ATP increased. The motor was now making ATP out of ADP.
The F
1
unit is coupled to an F
0
unit, which is stuck in the membrane of the mitochondria. Unless cranked by some other motor, the F
1
unit will
never synthesize ATP, it will only break it down. It needs to be coupled to a motor that cranks it in the counterclockwise direction—opposite the rotation during ATP breakdown. In our cells, this motor is contained in the F
0
subunit. The F
0
unit consists of several subunits, but overall, it consists of a ring of identical c-subunits, a large a-subunit, and two b-subunits, which reach up to hold the F
1
unit in place on top of the F
0
. Attached to the c-subunit ring is a flexible shaft (subunit γ), which transmits the rotation of the F
0
unit to the F
1
unit (
Figure 7.11
). The γ-subunit is asymmetrical. As it rotates, it alternately pushes on the b-subunit catalytic domains and changes the shape of the catalytic pocket to initiate the different stages of ADP to ATP processing. In other words, the catalytic site is adjustable to either accept ATP binding, ATP hydrolysis, or ADP release. The rotating shaft deforms each unit in turn to perform these steps in a sequential manner (
Figure 7.12
).
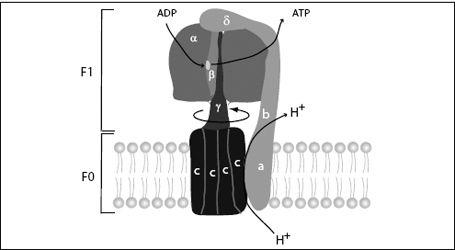
FIGURE 7.11.
The ATP synthase molecular electromotor and recharging station consists of the F
0
electromotor, which is embedded in the mitochondrial membrane, and F
1
, the rotary ADP recharging enzyme. F
0
consists of ten to fifteen c-subunits (depending on the organism) and an a-subunit and two b-subunits, which act as the stator. F
1
consists of three a-subunits and three b-subunits. The catalytic sites are on the b-subunit and are modified by the rotating shaft (γ-subunit) to perform the different steps of the ATP synthesis.
The F
0
motor is a biological electromotor. The voltage created by the electron transfer chain forces protons to flow back across the membrane.
However, they first have to flow through the F
0
motor. The c-subunits of the motor have sites that accommodate protons. As protons enter the membrane, they are attracted to these sites and stick there. This site interacts with a hydrophobic patch in the membrane, which repels the proton. This creates a tilted energy landscape, and by a Brownian ratchet mechanism, the motor rotates. This brings another c-subunit into a position to load a proton, and the cycle continues. As the F
0
unit rotates, each proton makes an almost complete circle before it arrives at a place where it is released and continues its journey across the membrane. When this motion is coupled to the F
1
unit of the synthase, the F
1
-ATPase is run in reverse and becomes an ATP
syn
thase, attaching phosphates to ADP.
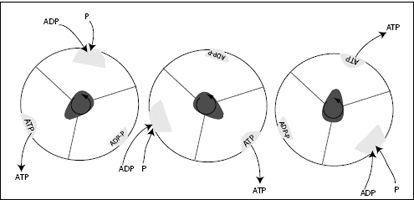
FIGURE 7.12.
The F
1
unit of ATP synthase seen from above, as the γ-subunit, or shaft, rotates counterclockwise. The shaft deforms the binding pocket in the identical three b-subunits such that at different times, either ADP and P bind, ADP and P are combined to ATP, or ATP is released. For every 360-degree rotation, this machine produces three ATP molecules.
GETTING AWAY WITH LESS
The F
0
subunit structure was even more difficult to decipher than the F
1
subunit structure. From early measurements, it was believed that four protons were required to rotate the machine 120 degrees and therefore produce one ATP molecule in the F
1
catalytic unit of the synthase. This would require the F
0
unit to contain 4 × 3, or 12 subunits (called c-subunits) that
drive motion when protons pass through. However, studies of ATP synthases in different organisms (ATP synthases are ubiquitous in everything from plants to amoeba to humans) showed that there was a variable number of c-subunits, depending on the species. Plants appeared to have 14 c-subunits, cyanobacteria 13–15, certain other bacteria 11, and, finally, yeasts and the bacterium
E. coli
(and, presumably, humans) 10. Since it is believed that each subunit uses one proton to initiate a rotation, this variable number of c-subunits suggests that there are variable numbers of protons needed to synthesize one ATP molecule. For example, if 14 c-subunits are present in plants, this would suggest that 14/3, or 4.7, protons are needed to make one ATP, but this does not seem the case from experiments. In plants, only 4 protons are needed. At the other extreme, 10 c-subunits would translate into 3.33 protons per ATP molecule, suggesting an improvement in efficiency. But this has not been proven, either. Many mysteries remain.
