Life's Ratchet: How Molecular Machines Extract Order from Chaos (28 page)
Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann

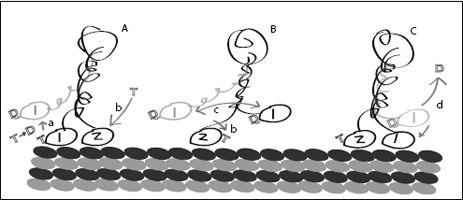
It seems we have returned to our original problem and must therefore add another step: The machine needs to reset itself to its original shape without creating backward motion. The machine needs a reset button, just like
Maxwell’s demon. Remember, Maxwell’s demon could only work if he received external energy to erase his memory, that is, if he received energy to reset.
For a machine moving along a molecular track, the reset step would consist of detaching from a track temporarily, releasing the control molecule, and resetting the machine’s shape. Then the machine would rebind to the track, bind to a control molecule, and push forward against the track—repeating the cycle. This idea leaves us with a few questions: Why doesn’t the motion of the machine become randomized during the reset step? That is, why does the detached machine not get swept away by the molecular storm? And how does the presence of a reset step help explain how molecular machines avoid violating the second law?
To try to answer these questions, let us turn to Sisyphus, the mythical character condemned by the gods (for a certain divinity-angering faux pas) to push a large, heavy boulder up a steep hill for eternity. If he could get the boulder to the top of the hill, his penance would be fulfilled. Alas, his boulder was cursed: Every time he approached the hilltop, the boulder slipped from his hands and rolled back down. What makes pushing a boulder so difficult? When we push something up an incline, we have to use enough force to overcome friction and gravity. As we apply this force and move the boulder over a distance, we perform work (in physics, work is the product of force and distance), and doing work requires energy.
Imagine if Sisyphus and his boulder were denizens of the nanoworld. If the boulder were nanosize, it would be continuously pushed around by the molecular storm. Ignoring sideways motion, the boulder would be randomly pushed up and down the hill (more often down than up, because up requires more energy). Sisyphus could adopt a very energy-efficient strategy to get the boulder to move uphill: simply wait for the boulder to move uphill by itself, randomly pushed by the molecular storm. But this is no good—the boulder is more likely to be pushed downhill than uphill.
What if Sisyphus steps in the way of the descending boulder? He could block the boulder’s downward motion, while allowing it to move uphill (
Figure 6.7
). He could repeat this all the way up the hill, as long as he quickly steps behind the boulder each time it makes a jump uphill. Now we are getting somewhere: The boulder is propelled by the molecular storm, but this random motion is rectified by Sisyphus’s blocking its motion
in the undesired direction. This is an extremely efficient way to move the boulder—a nano-Sisyphus would not have to push the boulder, and the boulder would move itself, driven by the molecular storm. Does this mean Sisyphus doesn’t need energy to move the boulder? No! Sisyphus still needs to move up the slope, step-by-step. These steps require energy.
How is this different from the ratchet in
Chapter 5
? The ratchet did not have a reset step and did not require energy. The ratchet was a passive device, which, by itself, was supposed to rectify thermal motion. But Sisyphus is different: He rectifies thermal motion at the expense of using a source of energy to reset the system (stepping up behind the boulder).
Let us take this analogy further. When nano-Sisyphus steps up the hill, he converts some source of free energy into kinetic energy. Then he stops. His kinetic energy is turned into heat (his sandals are hitting the ground). The act of stepping up the hill degrades free energy into heat. Sisyphus and the ground are getting a bit warmer. Now, whenever the boulder tries to move downward, it bounces off Sisyphus (who is warmer), and some energy is transferred to the boulder (which is colder). In the end, Sisyphus and the boulder end up at the same temperature (thermal equilibrium), but as a result, some of Sisyphus’s free energy ends up moving the boulder uphill, even though Sisyphus is not actually pushing the boulder!
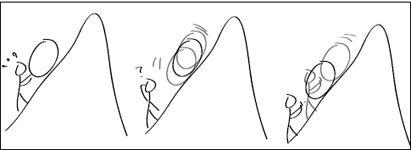
FIGURE 6.7.
Left: A macroscopic Sisyphus is condemned to sweat as he haplessly pushes the boulder uphill. Middle: A nanosize Sisyphus wonders how he can utilize the random thermal motion of the nanoboulder. Right: Nano-Sisyphus comes up with an idea: Step behind the boulder as it descends, but let it move freely when it happens to go uphill.
Moreover, this free energy is ultimately degraded into heat, as demanded by the second law of thermodynamics. We have found a way to
use the molecular storm to move the boulder without violating the second law. Without the chaotic motion of the molecular storm, the boulder would not move at all, and Sisyphus would be condemned to push forever. Yet, at the nanoscale, chaos can be turned into order, as long as we have a supply of free energy to periodically reset our machine.
The reset step, which we have found to be necessary for a molecular machine to work, can take many forms. This step is an example of a so-called
irreversible
step, because it degrades free energy into heat, and this heat cannot be turned back into free (usable) energy. Through this irreversible step, our molecular machine can extract energy from the molecular storm without violating the second law.
In hindsight, this makes sense: A reversible machine is a machine in thermodynamic equilibrium, with no irreversible steps. But if a machine is reversible, it can just as easily move one way or the other. If it can move just as easily forward as it can move backward, it cannot do any useful work. For a machine to do useful work, we need irreversibility. This was the missing something we were looking for in
Chapter 5
.
In
Chapter 3
, we found that living beings are open, dissipative, near-equilibrium systems. Now this statement takes on a new meaning: The irreversible steps needed to put our cellular machinery to work are paid for by a continuous supply of free energy. We must receive free (low-entropy) energy from the outside (food or sunlight), and this free energy is degraded (dissipated) by our molecular machines as they use it to harness the molecular storm.
Molecular machines need a supply of free energy. In some sense, they eat free energy. But how do they like their free energy served? On a bun with some ketchup? Joking aside, in animals, the free energy that feeds the molecular machines of cells comes from food. A bewildering network of enzymes in the stomach, intestines, and cells breaks down food as part of metabolism. The final product of this complicated process is a molecule called adenosine triphosphate, or ATP, the energy-storage molecule that brought myosin to life in the motility assay mentioned in
Chapter 4
. Three phosphates bind to adenosine to form ATP. With all three phosphates attached,
ATP is a bundle of concentrated energy. Snapping off one or two of the phosphate groups releases a great deal of energy—only the molecule’s activation barrier keeps the phosphates from detaching right away. But once ATP binds to a molecular machine, the phosphate groups snap off readily, ATP turns into ADP (adenosine
di
-phosphate), and the machine is provided with a large amount of energy.
What form does this energy take? It is vibrational energy; the release of the phosphate makes the enzyme shake and rattle. In some sense, we can think of it as local heating (higher temperature means more violent motion). The energy released by the loss of one phosphate is equivalent to heating the enzyme up to 7,000 degrees Fahrenheit. This additional shaking allows the molecular machine to overcome activation barriers that are otherwise unattainable.
Once the ATP is broken down to ADP, the ADP goes back to the cell’s recharging station, the mitochondrion. The mitochondrion is a cellular factory where sugar provides the energy to reattach phosphates to ADP, reconstituting ATP.
Righty tighty, lefty loosey
—G
OOD ADVICE WHEN PUTTING TOGETHER
I
KEA FURNITURE
Now that we know what is on a molecular machine’s menu, let us find out how real molecular machines implement the nano-Sisyphus method outlined above. As described earlier, nature has taken two major approaches to machine design: a robust (“super-Hummer”) strategy and a floppy (“If you can’t beat ’em, join ’em”) strategy. Scientists refer to these as tight and loose coupling, respectively. The boundaries between the two are not well defined and are vigorously debated in the scientific community. Each time a new molecular machine is identified, the tight-versus-loose debate flares up again, with both factions claiming the new machine for their camp. Before I try to weigh in on this debate, let me further explain the difference between the two mechanisms.
When biophysicists talk of tight coupling, they are referring to two (related) ideas. First, they think of a close coupling between the supplied ATP
and the work steps taken. Second, tight coupling refers to the machine’s being bound to a molecular track at all times. A tightly coupled motor uses one ATP molecule to make exactly one step while at least partially bound to a track. A loosely coupled machine, by contrast, may let go of the track from time to time. Detaching from the track allows a loosely coupled machine to make more than one step per ATP molecule, but it also allows the molecular storm to push the machine in an undesired direction.
As discussed earlier, in the super-Hummer model (i.e., a tightly coupled machine), there must be some minimal degree of letting go—otherwise the machine would not move at all. A good analogy is a human walking. In order to move forward, we have to break contact with the ground from time to time (even when we shuffle, we shift from firm contact to sliding). We can do this because we always keep one foot firmly planted on the ground, while moving the other. This is, in essence, how a number of molecular machines, or motors, work. For example, a molecular motor called kinesin—a fifty-nanometer-long assembly of protein molecules—walks on two feet (or
heads
, as biologists confusingly call them) on a molecular track called a microtubule, always keeping one foot planted to the track. These motors are used to move cargo throughout cells—they are nanosize Sherpas, carrying heavy molecular loads along a one-way track to distant regions of the cell (
Figure 6.8
).
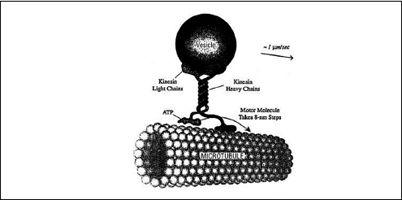
FIGURE 6.8.
A kinesin molecular motor carrying a vesicle filled with nutrients walks along a microtubule. The whole molecular motor is only about fifty nanometers in height. © 1999 Robert A. Freitas Jr.,
www.nanomedicine.com
. Used with permission.