Junk DNA: A Journey Through the Dark Matter of the Genome (18 page)
Read Junk DNA: A Journey Through the Dark Matter of the Genome Online
Authors: Nessa Carey

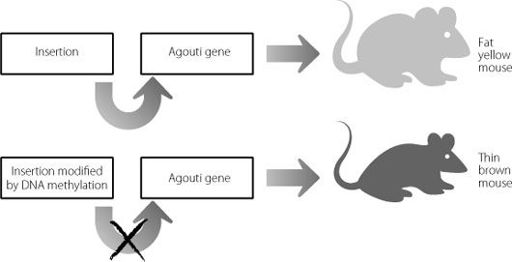
Figure 9.1
In the top panel an insertion drives expression of the
Agouti
gene, leading to a fat yellow mouse. In the bottom panel the insertion has been modified through DNA methylation. The insertion can no longer drive expression of the
Agouti
gene, and the mouse is brown and skinny.
Epigenetics and expansions
The cross-talk between epigenetics and junk DNA is also responsible for the impact of certain genetic mutations. The classic example is Fragile X syndrome, which was described in Chapters 1 and 2. The mutation that causes this condition is the expansion of the CCG triplet repeat, sometimes involving thousands of copies. This repeat contains a C followed by a G – the CpG formation introduced earlier in this chapter as the target sequence for DNA methylation. When this junk repeat sequence gets extremely large, it becomes irresistible to the enzymes and proteins that add a methyl group to the motif. This leads to very heavy methylation of the repeat, which in turn attracts all the proteins that repress gene expression, and even changes in the structure of the DNA itself. The end result is that it is impossible for the cell to express the Fragile X protein, and the consequence of this interaction between junk DNA and epigenetics is a lifetime of learning disability and social disadvantage.
Footnotes
a
The exceptions are the cells of the immune system that fight off specific infections. Unusually, these cells rearrange some of their genes to create different combinations of antibodies and receptors, able to respond to a vast range of foreign proteins.
b
The name for this major repressor enzyme is EZH2. It is responsible for adding three methyl molecules to an amino acid called lysine at position 27 on histone H3. The technical nomenclature for this modification is H3K27me3 and it is the best-characterised repressive mark in epigenetics outside of DNA methylation.
c
This complex is known as Polycomb Response Complex 2 or PRC2. The activity of PRC2 is closely coordinated with that of another repressive complex called PRC1. PRC2 usually establishes the first repressive modifications at a genomic region and PRC1 follows on with additional modifications that stabilise the repressive state.
10. Why Parents Love Junk
One of the first Bible stories learnt by children raised in the Judaeo-Christian faiths is the creation tale from Genesis. In this story, God creates the earth and the heavens and all that is in them, and finally he creates Adam and Eve. After that, peopling the earth is down to those two and their descendants, with no further divine intervention apart from the obvious exception in the Christian tradition at the start of the New Testament.
The strong grip of the Adam and Eve story perhaps drives or possibly reflects our ingrained acceptance of a simple piece of biology. To create a child you need a man and a woman. It’s not possible, biologically speaking, for a new child to be generated by two men, two women or a woman on her own.
This biological certainty seems so obvious that we virtually never think to question it. But we should, because sometimes the most extraordinary biology lies hidden in the most apparently mundane of assumptions. We should also question it because humans, like all other mammals who bear live young, are in the only class of the animal kingdom where there is never a virgin birth. The mammalian egg needs to be fertilised by a sperm in order to create a new individual. In every other class, there are examples of females who can give birth to live young without ever having mated. It’s not just restricted to the lower classes such as insects. Certain species of fish, amphibians, reptiles, and even some birds can do this. Mammals can’t, suggesting that this restriction on virgin birth arose relatively recently, following the
separation of the mammalian and reptilian lines over 300 million years ago.
We could speculate that this inability in mammals is more of an issue of delivery than fundamental biology. Perhaps two mammalian eggs can’t fuse, so they can’t create the zygote that can give rise to all other cells. As a consequence, mammalian reproduction would need a male donor because only a sperm can penetrate an egg and deliver its payload of DNA. It’s certainly true that mammalian eggs won’t normally fuse but this isn’t really the explanation. No, the explanation is far more interesting than this, and was demonstrated in a set of elegant experiments in the mid-1980s, using mice as the model system.
Researchers extracted mouse eggs that had been fertilised and took out the nucleus. They reconstituted the eggs using nuclei from eggs or sperm and implanted them back into the uterus of receptive female mice. The results are shown in Figure 10.1.
Live mice were only born after the eggs were reconstituted with a nucleus from both an egg and a sperm. If two sperm nuclei were used, or two egg nuclei, the embryos developed for a little while but then couldn’t develop any further. In genetic terms this is really peculiar. In all three experimental systems, the reconstituted egg contained the correct amount of DNA. In terms of DNA sequence there is no actual difference between the DNA from the sperm and the DNA from the egg, particularly because the experiments were designed so that the egg and the sperm each contributed an X chromosome. This created a strange paradox. In all three experimental situations, the DNA sequences involved were exactly the same. But live young only developed if those DNA sequences were donated by a male and a female.
1
We can be very confident that this requirement for both an egg and a sperm isn’t something restricted to mice, because of a human condition called a hydatidiform mole. A woman may appear to be pregnant, gaining weight and frequently suffering from extreme
morning sickness. But a scan will show the presence of an enlarged abnormal placenta, full of fluid-filled lumps, and no embryo. This is a hydatidiform mole and it is detected in about 1 in 1,200 pregnancies, although in some Asian populations this figure can reach 1 in 200. The structure will abort spontaneously at about four to five months post-fertilisation, although in societies with good prenatal care clinicians will remove it earlier to prevent the development of potentially dangerous tumours.
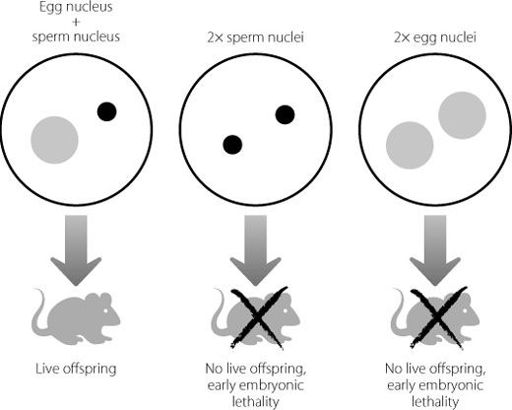
Figure 10.1
If an egg nucleus and a sperm nucleus are inserted into an empty egg which has lost its own nucleus, a live mouse is generated. If two egg nuclei or two sperm nuclei are used, the resulting embryos fail to develop properly. The same genetic information is present in all three scenarios.
Genetic analyses of the abnormal placenta have been very informative. They show that in most cases, hydatidiform moles arise when an egg that for some reason has no nucleus is penetrated by a sperm. The 23 chromosomes in the sperm are copied, to
create the normal human chromosome number of 46. In about a fifth of cases the mole is formed when two sperm penetrate one of the unusual nucleus-free eggs simultaneously, again generating the correct number of chromosomes. Just like the mouse experiments, the hydatidiform mole contains the correct number of chromosomes but they derive just from one parent, and this again leads to a severe failure in developmental pathways.
The clinical situation and the mouse experiments demonstrated something really fundamental. They showed that the gametes (eggs and sperm) contribute other information in addition to the genetic code. The findings simply can’t be explained by the DNA amount or sequence. At a phenomenon level, this is an example of epigenetics. We now know that at the molecular level the phenomenon is caused by the interaction of the epigenetic system and junk DNA.
Remembering where DNA comes from
Scientists have discovered that certain regions of DNA carry epigenetic modifications that indicate ‘I’m from mother’ or ‘I’m from father’. This is known as a parent-of-origin effect. In these regions of the genome, normal development is critically dependent on inheriting one copy of a specific gene (or genes) maternally and the other paternally.
The epigenetic modifications don’t just act as pieces of blue or pink genetic decoration indicating who gave you which copy of a gene. The modifications control the expression of specific genes, so that in each matching pair one will be turned on (for instance, the one you inherited from your father) but the one inherited from the other parent (in this case your mother) will be switched off. This system is known as imprinting, because the genes have been imprinted with information about their origin.
Normally, the fact that cells express two copies of a
protein-coding gene gives that cell a kind of insurance policy. Even if one of the copies suffers a mutation or perhaps is inappropriately silenced through abnormal epigenetic modifications, the cell has another copy to fall back on. But if the cell has switched off one of the copies through imprinting, this leaves it more vulnerable to the effects of a random shutdown of the other copy. The fact that for some genes the cell is willing to take this risk tells us that there must be substantial benefits to imprinting that outweigh this disadvantage.
It’s no accident that this system has only arisen in mammals. Female mammals make an extraordinarily large investment in the development of their offspring. They keep them inside their body, sharing nutrients with them via the placenta. Now, there are plenty of examples in other classes where a female invests in her young. Think of birds incubating their eggs, or crocodiles building elaborate nest piles and carefully regulating the temperature. But in no other class does the female actually nourish the developing embryos so dramatically.
But for good evolutionary reasons, there is a limit to this degree of maternal commitment. In terms of passing on her genes successfully, the female mammal would prefer to have more than one shot on goal. It’s possible that there may be other potential mates who are fitter (in evolutionary terms) than the one whose offspring she is carrying. So although she invests a lot in each pregnancy, it makes sense for the female to be able to breed more than once. There is a definite benefit to ensuring that the developing embryo or embryos gain enough nutrition from her that they have an excellent chance of surviving and reproducing themselves. But it doesn’t make sense to divert such a large amount of nutrition to the embryo that the mother ends up losing so much condition that she doesn’t survive or is subsequently infertile.
But the same isn’t really true for the male. It doesn’t really matter to him if his offspring draw so much nutrient from their
mother that she never reproduces again. In evolutionary terms, all he wants is for his offspring to be as well-nourished and strong as possible, so that they have the greatest chance of reaching sexual maturity and passing on his genes. He is likely to breed with other females, as relatively few mammals mate for life.
Female mammals can’t make decisions about the proportion of nourishment they pass on to the embryos in the uterus. They aren’t like birds who can abandon a nest. So evolution has reached an epigenetic stand-off in a nutritional arms race. Imprinting has evolved to balance out the competing demands of the male and female contributions to the genome. At a small number of genes, epigenetic modifications on the DNA inherited from the father set up patterns of gene expression that promote embryo growth. At the same genes, a different pattern of epigenetic modifications on the DNA inherited from the mother has the opposite effect.
During development, the relevant paternal genes often drive expression of a large, efficient placenta, as this is the organ that nourishes the embryos. That’s why in the hydatidiform moles, where all the genetic material is from the father, there is an abnormal and very large placenta.
Switching off by switching on
The number of imprinted protein-coding genes is fairly small, about 140 in mice.
2
They occur in clusters of between two and twelve genes and many of these clusters are quite similar to those in the human genome.
3
Perhaps unsurprisingly, the number of imprinted genes is much lower in marsupials where the young are only nourished in utero for a rather short period.
4
