Brilliant Blunders: From Darwin to Einstein - Colossal Mistakes by Great Scientists That Changed Our Understanding of Life and the Universe (26 page)
Authors: Mario Livio

The Estonian-Irish astronomer
Ernst Öpik proposed in 1951 that in the contracting cores of evolved stars (the stars themselves expand to become red giants), the temperature could reach a few hundred million degrees. At these temperatures, Öpik argued, most of the helium would fuse into carbon. Since, however, Öpik’s paper was published in the relatively little-known
Proceedings of the Royal Irish Academy,
not many astrophysicists knew about it.
Astrophysicist Edwin Salpeter, then at the beginning of his career at Cornell University, didn’t know about it either. In the summer of 1951, Salpeter was invited to visit the Kellogg Radiation Laboratory at Caltech, where the ebullient nuclear astrophysicist Willy Fowler and his group were becoming deeply involved in the study of nuclear reactions thought to be important for astrophysics. Starting with the same idea as Öpik,
Salpeter examined the triple alpha process in the hot inferno at the centers of red giants—precisely the problem abandoned by Hoyle’s graduate student. Salpeter immediately recognized that three helium nuclei could hardly be expected to collide simultaneously. It was more likely that two of them might stick together long enough to be struck by a third. Salpeter soon found that carbon could perhaps be produced via a low-probability, two-step process. In the first step, two alpha particles could combine to form a highly unstable isotope of beryllium (
8
Be), and in the second, the beryllium could capture a third alpha particle to form carbon. But there was still a serious problem. Experiments had shown that this particular isotope of beryllium disintegrates back into two alpha particles, with a fleeting mean lifetime of only about 10
-16
seconds (0.00 . . . 1 at the sixteenth decimal place). The question was whether at a temperature of over one hundred million Kelvin, the reaction rate could become so high that some of these ephemeral beryllium nuclei could fuse with the third helium nucleus before falling apart.
When he read Salpeter’s paper, Hoyle’s first reaction was anger with himself for having let such an important calculation slip through his fingers because of the mishap with the graduate student.
Upon a closer examination of the entire nuclear reactions network, however, Hoyle estimated that under Salpeter’s assumptions, all the carbon would be transformed into oxygen essentially as fast as it was produced, by fusing with yet another helium nucleus. Some thirty years later, he described this important realization:
“Bad luck for poor old Ed, I thought to myself.” (Ed Salpeter was, in fact, nine years younger than Hoyle.) But did this spell disaster for the entire scheme? These were precisely the types of situations in which Hoyle revealed his incredible physical intuition and the clarity of his thought. He started with the obvious: “There has to be some way of synthesizing
12
C.” After all, not only was carbon relatively abundant in the universe, but carbon was also crucial for life. After evaluating all the potential reactions in his head, Hoyle concluded: “Nothing was better than 3α.” So how could the carbon be prevented from slipping away into oxygen? In Hoyle’s mind, there was only one way: “
3α
had to go a lot faster than it had been calculated to do
[emphasis added].” In other words, beryllium and helium had to be able to fuse together so easily and so quickly that carbon would be produced at a much faster rate than it was destroyed. But what could substantially speed up the rate of carbon synthesis? Nuclear physicists knew of one thing: a “resonant state” in the carbon nucleus. Resonant states are values of the energy at which the probability for a reaction reaches a peak. Hoyle realized that if the carbon nucleus happened to have an energy level that perfectly matched the energy equivalent of the combined masses of the beryllium nucleus and an alpha particle (plus their kinetic energy of motion), then the rate for the fusion of beryllium with an alpha would increase significantly. That is, the probability for the unstable beryllium nucleus to absorb another helium nucleus (alpha particle) to form carbon would be enhanced greatly. But Hoyle did more than merely point out that a resonance would help. He calculated
precisely
the necessary energy level in the carbon nucleus to obtain the desired effect. Nuclear physicists measure energies in nuclei in units called MeV (an MeV is one million electron volts).
Hoyle calculated that for carbon production to match the observed cosmic abundance, a resonant state in
12
C was
needed, at about 7.68 MeV above the lowest energy level (the ground state) of the carbon nucleus. Furthermore, using the known symmetry of the
8
Be and
4
He nuclei, he predicted the quantum mechanical properties of this resonant state.
This was all very impressive, except for one “small” problem: No such state was known to exist! The mere idea that Hoyle would be using general astrophysical evidence to make an extremely precise prediction in nuclear physics (much more precise, in fact, than could be calculated based on nuclear physics) was nothing short of preposterous, but Hoyle never lacked chutzpah.
The time was January 1953, and Hoyle was spending a sabbatical of a few months at Caltech. Armed with his new prediction for an unknown energy level of the carbon nucleus, Hoyle went straight into Willy Fowler’s office at Kellogg Laboratory to see whether Fowler and his group could run experiments to verify the prediction.
What happened at that meeting has become legendary. Fowler recalled, “
Here was this funny little man who thought that we should stop all this important work that we were doing otherwise and look for this state, and we kind of gave him the brushoff. Get away from us, young fellow, you bother us.”
Hoyle himself remembered the meeting in a more positive light:
To my surprise, Willy didn’t laugh when I explained the difficulty. I cannot remember whether he called in a Kellogg mob [the nuclear physics group that included, among others, Ward Whaling, William Wenzel, Noel Dunbar, Charles Barnes, and Ralph Pixley] there and then, or whether it was a few hours or a day or two later . . . It was then that general consensus decided a new experiment should be done.
In an interview in 2001, neither Ward Whaling nor Noel Dunbar remembered any specific details of this meeting, but Charles Barnes recalled that Willy’s rather small office was packed and that “as Fred presented his ideas, it was clear that the audience was visibly skeptical. Even Willy seemed to be somewhat skeptical.” Whatever
precisely happened at that meeting, the net result was that the “Kellogg mob” did decide to perform the experiment, and
Ward Whaling and his colleagues were identified as the group that had the best experimental setup to perform the necessary measurements.
Whaling, Dunbar, and their collaborators decided to tackle the problem by bombarding nitrogen (
14
N) nuclei with deuterium (
2
H). This nuclear reaction produces carbon (
12
C) nuclei and alpha particles (
4
He). By examining carefully the energy of the outstreaming alpha particles (and remembering that the total energy is conserved), they could detect not only particles coming out with high energy (therefore leaving the carbon in its low-energy ground state) but also particles emerging with lower energy, indicating that some energy was left in the carbon nucleus. The results were clear. Within a couple of weeks, the experimental group found a resonance in carbon at 7.68 MeV (with a possible error of 0.03 MeV)—in incredible agreement with Hoyle’s prediction!
In their just-over-one-page paper describing the results, the nuclear physicists started by noting: “Hoyle explains the original formation of elements heavier than helium by this process” (fusion of beryllium with helium). They finished with an acknowledgement: “We are indebted to Professor Hoyle for pointing out to us the astrophysical significance of this level.”
Despite his amazingly successful prediction, Hoyle realized that this was not the time to rest on his laurels. For carbon to survive, the nuclei had to obey yet another important requirement: Carbon had to be unable to rapidly capture a fourth alpha particle that would have transformed it all into oxygen. In other words, one had to be sure that there is no resonant state in the oxygen nucleus to enhance the carbon-plus-alpha reaction rate. To complete his triumph with the theory of carbon production, Hoyle showed that such a resonant reaction indeed does not occur—the energy of the respective level in oxygen is lower by about 1 percent from the value that would have made it resonant.
One might have thought that with such a coup under his belt, Hoyle would immediately rush to announce it to the world. In
reality,
more than a half year passed from the confirmation of his prediction until Hoyle reported on it briefly at a meeting of the American Physical Society in Albuquerque. Even in subsequent years, Hoyle never made a big deal of his remarkable achievement. In 1986 he commented:
In a sense this was but a minor detail. But because it was seen by physicists as an unusual and successful prediction it had a disproportionate effect in converting them from the currently held view that the elements were all synthesized in the early moments of a hot universe to the more mundane view that the elements are synthesized in stars.
Others did not think that this was “but a minor detail.” When the boisterous George Gamow came to summarize his own views on Hoyle’s role in the theory of the formation of the elements, he did so by a witty account that he entitled “New Genesis”:
In the beginning God created radiation and ylem. And ylem was without shape or number, and the nucleons were rushing madly over the face of the deep. And God said: “Let there be mass two.” And there was mass two. And God saw deuterium, and it was good. And God said: “Let there be mass three.” And God saw tritium and tralphium [Gamow’s nickname for the isotope of helium
3
He] and they were good. And God continued to call number after number until He came to the transuranium elements. But when He looked back on his work He found that it was not good. In the excitement of counting, He missed calling for mass five and so, naturally, no heavier elements could have been formed. God was very much disappointed, and wanted first to contract the universe again, and to start all over from the beginning. But it would be much too simple. Thus being almighty, God decided to correct His mistake in a most impossible way.
And God said: “Let there be Hoyle.” And there was Hoyle. And God looked at Hoyle . . . and told him to make heavy elements in any way he pleased. And Hoyle decided to make heavy elements in stars, and to spread them around by supernovae explosions. But in doing so he had to obtain the same abundance curve which would have resulted from nucleosynthesis in ylem, if God would not have forgotten to call for mass five. And so, with the help of God, Hoyle made heavy elements in this way, but it was so complicated that nowadays neither Hoyle, nor God, nor anybody else can figure out exactly how it was done.
Note, by the way, that according to this “New Genesis,” even God made a blunder!
The Royal Swedish Academy of Sciences also did not think that Hoyle’s prediction was merely a minor detail. In 1997 it decided to give the prestigious Crafoord Prize (awarded in disciplines chosen to complement those for which the Nobel prizes are given) to Hoyle and Salpeter “for their pioneering contribution to the study of nuclear processes in stars and stellar evolution.” In their announcement of the prize, the academy noted:
“Perhaps his [Hoyle’s] most important single contribution within this field was a paper where he demonstrated that the existence of carbon in Nature implied the existence of a certain excited state in the carbon nuclei above the ground state. This prediction was later verified experimentally.”
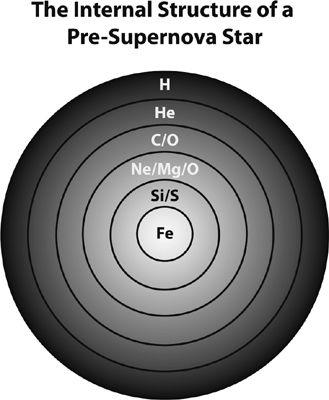
Figure 21
Hoyle followed up on his prediction for the carbon level with a paper that established the foundation for the theory of nucleosynthesis in stars: the concept that most chemical elements and their isotopes were synthesized from hydrogen and helium by nuclear reactions within massive stars.
In this paper, published in 1954, Hoyle explained how the abundances of heavy elements today are the direct products of
stellar evolution
. Stars spend their lives in a continuous battle against gravity. In the absence of an opposing force, gravity would cause any star to collapse to its center. By “igniting” nuclear reactions in their cores, stars create extremely high
temperatures, and the associated high pressures support the stars against their own weight. Hoyle described how after each central nuclear fuel is consumed (first, hydrogen is fused into helium, then helium into carbon, then carbon into oxygen, and so on), gravitational contraction causes the temperature in the core to increase until the “ignition” of the next nuclear reaction. This way, Hoyle reasoned, new elements are synthesized, all the way up to iron, in each successive core-burning episode. Since each burning core is smaller than the preceding one, the star develops an onionskin-like structure, in which each layer is composed of the main product—“ashes,” if you like, of the preceding nuclear reaction (figure 21). Since iron is the most stable nucleus, once an iron core forms, no more nuclear energy is available from fusion of nuclei into heavier ones. Without a source of internal heat to combat gravity, the stellar core collapses, triggering a dramatic explosion. These so-called supernova explosions powerfully eject all the forged elements into interstellar space, where they enrich the gas from which later generations of stars and planets form. The temperatures attained during the explosions are so high that elements heavier than iron are formed by neutrons bombarding the stellar material. Hoyle’s scenario remains to this day the broad picture depicting the evolution of stars. Surprisingly, this key paper in the development of the theory of stellar nucleosynthesis received relatively little attention at the time, perhaps because it was published in a new astrophysical journal that was relatively unknown to the nuclear physics community.
