What If? (6 page)
Authors: Randall Munroe

Our light is definitely visible, so we’ve accomplished our goal! Good job, team.
Well . . .
Th
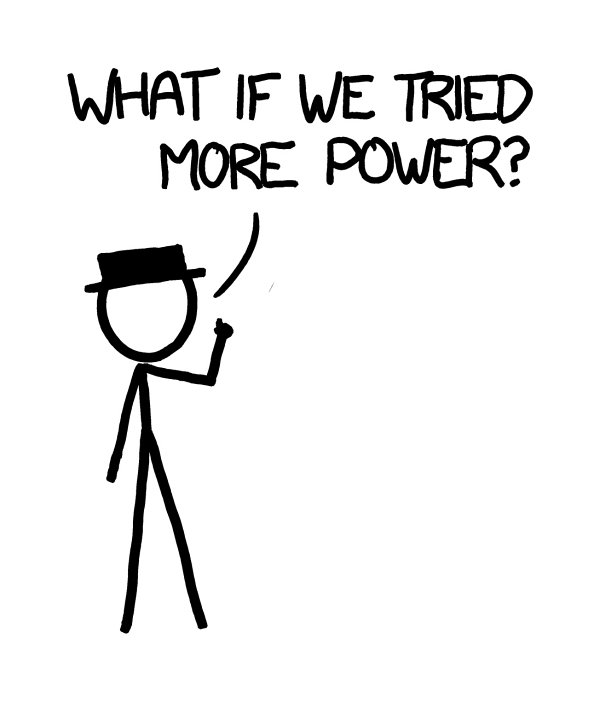
e Department of Defense has developed megawatt lasers, designed for destroying incoming missiles in mid-flight.
Th
e Boeing YAL-1 was a megawatt-class chemical oxygen iodine laser mounted in a 747. It was an infrared laser, so it wasn’t directly visible, but we can imagine building a visible-light laser with similar power.
Finally, we’ve managed to match the brightness of sunlight!
We’re also drawing 5 petawatts of power, which is double the world’s average electricity consumption.
Okay, let’s mount a megawatt laser on every square meter of Asia’s surface. Powering this array of 50 trillion lasers would use up Earth’s oil reserves in approximately two minutes, but for those two minutes, the Moon would look like this:
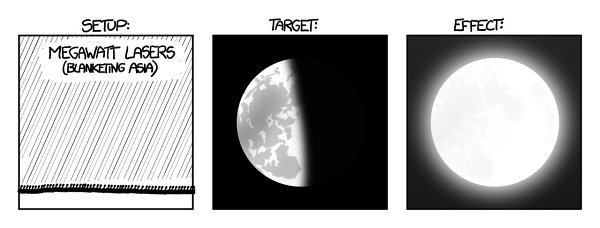
Th
e Moon would shine as brightly as the midmorning sun, and by the end of the two minutes, the lunar regolith would be heated to a glow.
Okay, let’s step even more firmly outside the realm of plausibility.
Th
e most powerful laser on Earth is the confinement beam at the National Ignition Facility, a fusion research laboratory. It’s an ultraviolet laser with an output of 500 terawatts. However, it fires only in single pulses lasting a few nanoseconds, so the total energy delivered is equivalent to about a quarter-cup
of gasoline.
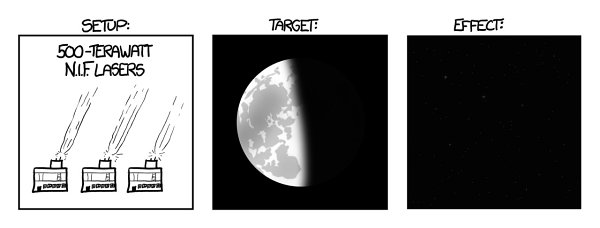
Let’s imagine we somehow found a way to power and fire it continuously, gave one to everyone, and pointed them all at the Moon. Unfortunately, the laser energy flow would turn the atmosphere to plasma, instantly igniting the Earth’s surface and killing us all. But let’s assume that the lasers somehow pass through the atmosphere without interacting.
Under those circumstances,
it turns out Earth would
still
catch fire.
Th
e reflected light from the Moon would be four thousand times brighter than the noonday sun. Moonlight would become bright enough to boil away Earth’s oceans in less than a year.
But forget the Earth
—
what would happen to the Moon?
Th
e laser itself would exert enough radiation pressure to accelerate the Moon at about one ten millionth of a gee.
Th
is acceleration wouldn’t be noticeable in the short term, but over the years, it would add up to enough to push it free from Earth orbit . . .
. . . if radiation pressure were the only force involved.
Forty megajoules of energy is enough to vaporize a kilogram of rock. Assuming Moon rocks have an average density of about 3 kg/liter, the lasers would pump out enough energy to vaporize
4 meters of lunar bedrock per second:
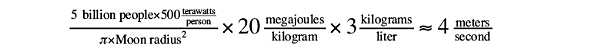
However, the actual lunar rock wouldn’t evaporate that fast
—
for a reason that turns out to be very important.
When a chunk of rock is vaporized, it doesn’t just disappear.
Th
e surface layer of the Moon becomes a plasma,
but that plasma would still block the path of the beam.
Our laser would keep pouring more and more energy into the plasma, and the plasma would keep getting hotter and hotter.
Th
e particles would bounce off each other, slam into the surface of the Moon, and eventually blast into space at a terrific speed.
Th
is flow of material effectively turns the entire surface of the Moon into a rocket
engine
—
and a surprisingly efficient one, too. Using lasers to blast off surface material like this is called laser ablation, and it turns out to be a promising method for spacecraft propulsion.
Th
e Moon is massive, but slowly and surely the rock plasma jet would begin to push it away from the Earth. (
Th
e jet would also scour the face of the Earth clean and destroy the lasers, but we’re pretending
that they’re invulnerable.)
Th
e plasma would also physically tear away the lunar surface, a complicated interaction that’s tricky to model.
But if we make the wild guess that the particles in the plasma exit at an average speed of 500 kilometers per second, then it will take a few months for the Moon to be pushed out of range of our laser. It would keep most of its mass, but escape Earth’s
gravity and enter a lopsided orbit around the sun.
Technically, the Moon wouldn’t become a new planet, under the IAU definition of a planet. Since its new orbit would cross Earth’s, it would be considered a dwarf planet like Pluto.
Th
is Earth-crossing orbit would lead to periodic unpredictable orbital perturbation. Eventually it would either be slingshotted into the Sun, ejected toward the
outer solar system, or slammed into one of the planets
—
quite possibly ours. I think we can all agree that in this case, we’d deserve it.
Scorecard:
And that, at last, would be enough power.
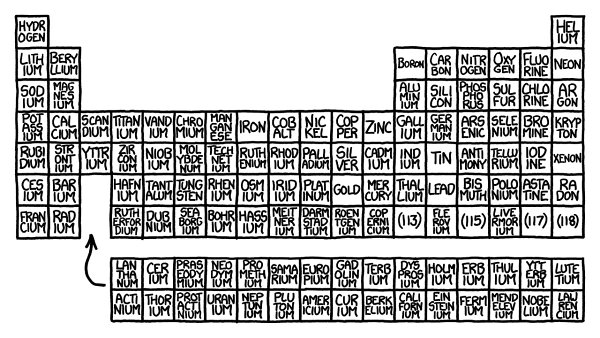
Periodic Wall of the Elements
Q.
What would happen if you made a periodic table out of cube-shaped bricks, where each brick was made of the corresponding element?
—Andy Connolly
A.
Th
ere are people who
collect elements.
Th
ese collectors try to gather physical samples of as many of the elements as possible into periodic-table-shaped display cases.
1
Of the 118 elements, 30 of
them
—
like helium, carbon, aluminum, iron, and ammonia
—
can be bought in pure form in local retail stores. Another few dozen can be scavenged by taking things apart (you can find tiny americium samples in smoke detectors). Others can be ordered over the Internet.
All in all, it’s possible to get samples of about 80 of the elements
—
90, if you’re willing to take some risks with your health, safety,
and arrest record.
Th
e rest are too radioactive or short-lived to collect more than a few atoms of them at once.
But what if you
did
?
Th
e periodic table of the elements has seven rows.
2
- You could stack the top two rows without much trouble.
- Th
e third row would burn you with fire. - Th
e fourth row would kill you with toxic smoke. - Th
e fifth row would do all that stuff PLUS give you a mild dose of radiation. - Th
e sixth row would explode violently, destroying the building in a cloud of radioactive, poisonous fire and dust. - Do not build the seventh
row.
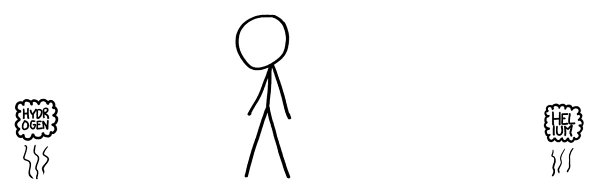
We’ll start from the top.
Th
e first row is simple, if boring:
Th
e cube of hydrogen would rise upward and disperse, like a balloon without a balloon.
Th
e same goes for helium.
Th
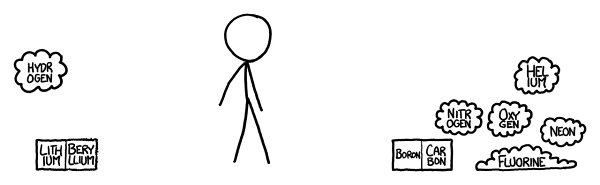
e second row is trickier.
Th
e lithium would immediately tarnish.
Th
e beryllium is pretty toxic, so you should handle it carefully and avoid getting any dust in the air.
Th
e oxygen and nitrogen drift around, slowly dispersing.
Th
e neon floats away.
3
Th
e pale yellow fluorine gas would spread across the ground. Fluorine is the most reactive, corrosive element in the periodic table. Almost any substance
exposed to pure fluorine will spontaneously catch fire.
I spoke to organic chemist Derek Lowe about this scenario.
4
He said that the fluorine wouldn’t react with the neon, and “would observe a sort of armed truce with the chlorine, but everything else, sheesh.” Even with the later rows, the fluorine would cause problems as it spread, and if it came in contact with any moisture, it would form
corrosive hydrofluoric acid.
If you breathed even a trace amount, it would seriously damage or destroy your nose, lungs, mouth, eyes, and eventually the rest of you. You would definitely need a gas mask. Keep in mind that fluorine eats through a lot of potential mask materials, so you would want to test it first. Have fun!
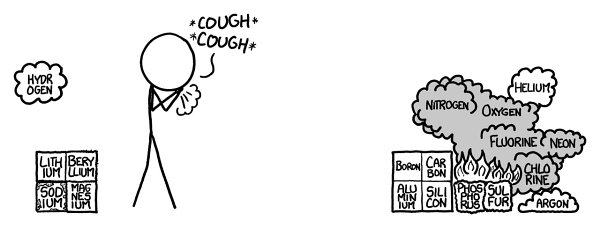
On to the third row!
Half of the data here is from the
CRC Handbook of Chemistry and Physics
and the other half is from
Look Around You
.
Th
e big troublemaker here is phosphorus. Pure phosphorus comes in several forms. Red phosphorus is reasonably safe to handle. White phosphorus spontaneously ignites on contact with air. It burns with hot, hard-to-extinguish flames and is, in addition, quite poisonous.
5
Th
e sulfur wouldn’t be a problem under normal circumstances; at worst, it would smell bad. However, our sulfur is sandwiched between burning phosphorus on the left . . . and the fluorine and chlorine on the right. When exposed to pure fluorine gas, sulfur
—
like many substances
—
catches fire.
Th
e inert argon is heavier than air, so it would just spread out and cover the ground. Don’t worry
about the argon. You have bigger problems.
Th
e fire would produce all kinds of terrifying chemicals with names like sulfur hexafluoride. If you’re doing this inside, you’d be choked by toxic smoke and your building might burn down.
And that’s only row three. On to row four!
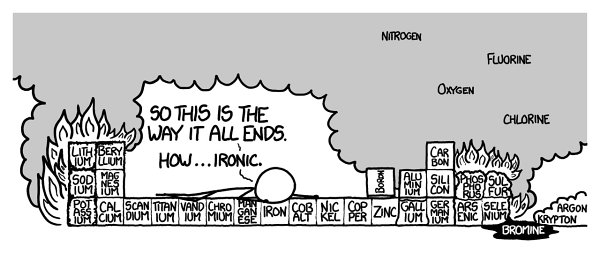
“Arsenic” sounds scary.
Th
e reason it sounds scary is a good one: It’s toxic to virtually all forms of complex life.
Sometimes this kind of panic over scary chemicals is disproportionate; there are trace amounts of natural arsenic in all our food and water, and we handle those fine.
Th
is is not one of those times.
Th
e burning phosphorus (now joined by burning potassium,
which is similarly prone to spontaneous combustion) could ignite the arsenic, releasing large amounts of arsenic trioxide.
Th
at stuff is pretty toxic. Don’t inhale.
Th
is row would also produce hideous odors.
Th
e selenium and bromine would react vigorously, and Lowe says that burning selenium “can make sulfur smell like Chanel.”
If the aluminum survived the fire, a strange thing would happen
to it.
Th
e melting gallium under it would soak into the aluminum, disrupting its structure and causing it to become as soft and weak as wet paper.
6
Th
e burning sulfur would spill into the bromine. Bromine is liquid at room temperature, a property it shares with only one other element
—
mercury. It’s also pretty nasty stuff.
Th
e range of toxic compounds that would be produced by this blaze is,
at this point, incalculably large. However, if you did this experiment from a safe distance, you might survive.
Th
e fifth row contains something interesting: technetium-99, our first radioactive brick.
Technetium is the lowest-numbered element that has no stable isotopes.
Th
e dose from a 1-liter cube of the metal wouldn’t be enough to be lethal in our experiment, but it’s still substantial.
If you spent all day wearing it as a hat
—
or breathed it in as dust
—
it could definitely kill you.
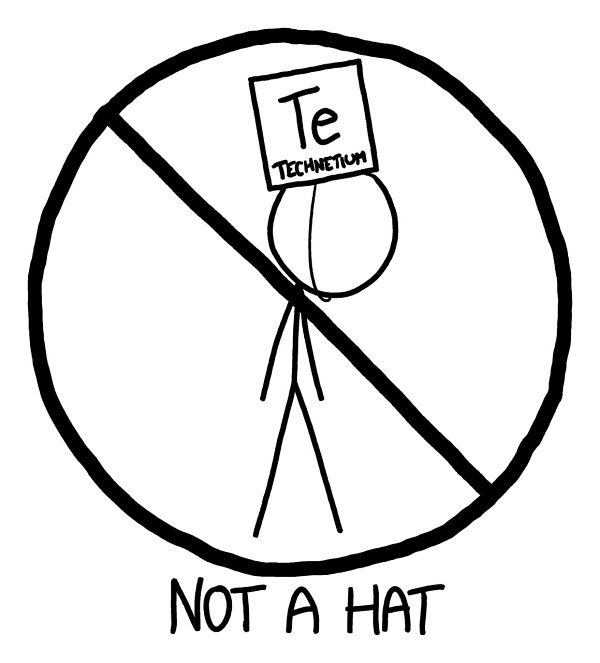
Techneteium aside, the fifth row would be a lot like the fourth.
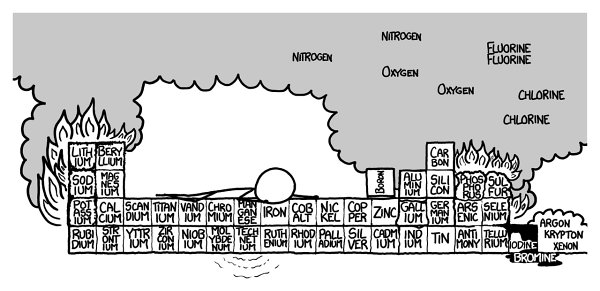
On to the sixth row! No matter how careful you are, the sixth row would definitely kill you.