The Flamingo’s Smile (32 page)
Read The Flamingo’s Smile Online
Authors: Stephen Jay Gould

The theoretical source of Darwin’s error lies in a fairly arcane principle of the creationist style of taxonomy that he followed. If animals are created according to a rational and general plan in the Deity’s mind, then certain “key” characters might be clues to taxonomic structure at different levels. For example, variation in such “superficial” characters as size and shape might define different species, while variation in such “fundamental” traits as the form of essential organs might record the more important differences between genera and families. Ideally, a hierarchy of key characters should define taxonomic levels. Darwin tried to follow such a system in his preliminary
Beagle
classifications. Species within a bird genus should differ in plumage, while genera should be separated by such characters as the form of the beak. Darwin’s finches are all similar in plumage, but differ greatly in styles of feeding and, consequently, in the shapes of their beaks. By Darwin’s creationist hierarchy of key characters, they belonged to different genera or families.
The key character hierarchy makes no sense in an evolutionary context. Characters that define genera in one situation might vary widely among species within another group. Bills may define feeding types, and feeding types may usually distinguish genera on continents. But if only one kind of small bird manages to reach an oceanic archipelago and then diversifies, in the absence of competitors, into a wide range of niches and feeding types, then the traditional criterion for genera—form of the bill—will now differ among closely related species. In the blooming and buzzing confusion of evolution, as opposed to the order of a creator’s mind, responses to local preset environments, not rules of change, determine what parts of the body will be modified in any particular case. Behavior and plumage in one place; feeding and shape of the beak in another. There is no such thing as an invariably “specific” or “generic” character.
In summary, then, Darwin entered and left the Galápagos as a creationist, and his style of collection throughout the visit reflected his theoretical stance. Several months later, compiling his notes at sea during the long hours of a Pacific crossing, he briefly flirted with evolution while thinking about tortoises and mockingbirds, not finches. But he rejected this heresy and docked in England, October 2, 1836, as a creationist harboring nascent doubts.
This retelling of the finch story is particularly satisfying because the new version squares so much better than the old legend with Darwin’s use of the Galápagos finches throughout his later writing. He never mentioned them in any of the four
Transmutation Notebooks
, which he kept from 1837 to 1839 and which serve as a foundation for his later work. They receive only passing notice in the first (1839) edition of the
Voyage of the Beagle
. To be sure, the second edition (1845) does contain this prophetic statement, written after Darwin had learned that the finches form a closely knit genealogical group.
Seeing this gradation and diversity of structure in one small, intimately related group of birds, one might really fancy that from an original paucity of birds in this archipelago, one species had been taken and modified for different ends.
But if the finches made such a belated impression, the impact didn’t seem to last. Darwin’s finches are not mentioned at all in the
Origin of Species
(1859); the ornithological star of that great book is the domesticated pigeon. Sulloway concludes, rightly I think:
Contrary to the legend, Darwin’s finches do not appear to have inspired his earliest theoretical views on evolution, even after he finally became an evolutionist in 1837; rather it was his evolutionary views that allowed him, retrospectively, to understand the complex case of the finches.
Darwin returned to England in 1836 as an ambitious young man, anxious to make his mark in science; his later, courtly modesty as an old man should not be allowed to mask this youthful vigor. He knew that the key to his reputation lay in the valuable specimens he had collected on the
Beagle
, and therefore he made determined and successful efforts to farm them out to the best specialists and to procure funds for publication of the results. In March 1837 he moved to London, largely to be near the various experts studying his specimens. He began a series of meetings with these men, finally learned the true character of his material, and emerged within a month or two as an evolutionist.
He wrote, in the famous entry in his pocket journal cited earlier, that the character of South American fossils and species of the Galápagos had been the primary catalysts of his evolutionary conversion. Richard Owen, Britain’s most eminent vertebrate paleontologist, had agreed to study the fossils and informed Darwin that they represented different, usually larger versions of distinctive animals that still inhabit South America. Darwin recognized that the best interpretation of this “law of succession” cast the ancient forms as evolutionary ancestors of altered modern animals.
The famous ornithologist John Gould (no relation) had taken charge of the
Beagle
’s birds. Darwin met with him toward the middle of March and learned that the three forms of mockingbirds were separate species, not mere and superficial varieties of a single, created type. Darwin had already proclaimed that such a conclusion (which he had previously rejected) “would undermine the stability of species.” Moreover, Gould informed him that twenty-five of his twenty-six Galápagos land birds were new species, but clearly allied to related forms on the South American mainland. Darwin integrated this spatial information with the temporal data that Owen had supplied, and he tilted further toward evolution. The distinct Galápagos birds must be evolutionary descendants of mainland colonists from South America. Darwin was now fully primed for an evolutionary reading of the finches, and Gould’s correction of Darwin’s errors furnished this piece of the puzzle as well (although Gould himself did not adopt evolutionary views).
Although a creationist in taxonomy, Gould recognized right away that bills could not be used as a key character to separate genera of Galápagos finches. He understood that these birds were not, as Darwin had thought, a heterogeneous assemblage of divergent finches with an unrelated warbler and oriole thrown in, but a peculiar group of thirteen closely related species, which he placed in a single genus with three subgenera. “The bill appears to form only a secondary character,” Gould proclaimed. Darwin finally had the basis of an evolutionary story.
Darwin was exhilarated as he converted to evolution and prepared to reread his entire voyage in this new light. But he was also acutely embarrassed because he now realized that his failure to separate finches by islands, no particular problem in a creationist context, had been a serious and lamentable lapse. He couldn’t do much with his own collection, beyond probing a faulty and fading memory; but fortunately, three of his shipmates had also collected finches—and since they (ironically) had not collected with an actively creationist theory in mind (with its implied irrelevancy for precise geographic data), they had recorded the islands of collection. As a further irony, one of these collections had been made by Captain Fitzroy himself, later Darwin’s implacable foe and the man who stalked around the British Association meeting where Huxley demolished Wilberforce, holding a Bible above his head and exclaiming, “the Book, the Book.” (Fitzroy’s collection included twenty-one finches, all labeled by island. Darwin also had access to the smaller collections of his servant Syms Covington and of Harry Fuller, who had spent a week collecting with him on James Island.)
Darwin therefore tried to reconstruct the localities of his own specimens by comparing them with the accurately labeled collections of his shipmates and, unfortunately as it turned out, by assuming that the finch story would resemble the pattern of the mockingbirds—with each species confined to a definite island. But since most of the finch species inhabit several islands, this procedure produced a large number of errors. Sulloway reports that substantial doubt still exists about the accuracy of geographic information for eight of fifteen among Darwin’s “type” (or name bearing) specimens of finches. No wonder he was never able to make a clear and coherent story of Darwin’s finches. No wonder, perhaps, that they never even appeared in the
Origin of Species
.
Why, in conclusion, is this correction of the finch legend of any great importance? Are the two stories really all that different? Darwin, in either case, was greatly influenced by evidence from the Galápagos. In the first, and false, version he understands it all by himself while on the visit. In the second, modified account he requires a nudge (and some substantial corrections) from his friends when he returns to London.
I find a world of difference between the tales for what they imply about the nature of creativity. The first (false) version upholds the romantic and empirical view that genius attains its status from an ability to see nature through eyes unclouded by the prejudices of surrounding culture and philosophical presupposition. The vision of such pure and unsullied brilliance has nurtured most legends in the history of science and purveys seriously false views about the process of scientific thought. Human beings cannot escape their presuppositions and see “purely” Darwin functioned as an active creationist all through the
Beagle
voyage. Creativity is not an escape from culture but a unique use of its opportunities combined with a clever end run around its constraints. Scientific accomplishment is also a communal activity, not a hermit’s achievement. Where would Darwin have been in 1837 without Gould, Owen, and the active scientific life of London and Cambridge?
Once we abandon the alluring, but fallacious, image of Darwin winning his intellectual battle utterly alone at sea, we can ask the really interesting question that begins to probe Darwin’s particular genius. Gould was the expert. Gould resolved the details correctly. Gould, a staunch creationist in taxonomy, nonetheless recognized that he had to abandon beaks as key characters. Darwin could accomplish none of this. But Darwin, not Gould, recognized that all the pieces required a stunningly new explanation—evolution—to make a coherent story. The amateur triumphed when the stakes were highest, while the professional got the details right and missed the organizing theme.
Darwin continued to work in this way throughout his career. Somehow, as an amateur, he could cut through older patterns of thought to glimpse new modes of explanation that might better fit an emerging, detailed story constructed by experts who, somehow, could not take the big and final step. But Darwin worked with his culture and with his colleagues. Science is a collective endeavor, but some individuals operate with an enlarged vision—and we would like to know how and why. We can ask no harder question, and I propose no general solution. But we do need to clear away heroic legends before we can begin.
SINCE IT WAS
only a few miles to Tipperary, not the long way of song and legend, I took a detour to visit the town. Soon I felt like the city slicker of that old New England joke. Looking for a small town, he stops before a general store and asks an old-timer, “Where is Pleasantville?” “Don’t you move a god-damned inch,” comes the reply.
Tipperary, made large by its fame and my imagination, is but one main street with a few stores and houses. This eerie scene repeated itself again and again during my visit to this most beautiful of European lands. For Ireland, contrary to the trend of most other countries, is a depopulated nation. Its current count of some 3 million inhabitants includes but half of the 1840 total. Abandoned homes, farms, and even towns lie strewn about the countryside.
The beginning of the great emigration that so enriched my native city of New York and my current Boston home dates to the great potato famines of 1845 and 1846, when half a million people starved to death and another million left. The potato is a remarkable food. It contains so well balanced an array of nutrients that people can live on virtually nothing else for years on end. Monotonous perhaps, spuds being spuds, but quite viable. Irish peasants often ate nothing but potatoes through the long winter months. But disease attacked the crop in 1845 and virtually destroyed it, producing unprecedented starvation and the great exodus to Liverpool and beyond.
The Irish potato blight illustrates a classic dilemma in agriculture. To produce the “best” plant for maximal yields, farmers and scientists will hone and select for many generations until they obtain just the right combination of features. They will then propagate their entire crop from this improved form. These plants, as offspring of a single parental type, are genetically uniform and depleted in variability. In other words, we trade genetic diversity for an unvarying optimum.
All may be well for a while, but uniform stocks are exquisitely susceptible to the ravages of disease. If some virus, bacterium, or fungus successfully attacks the plants, it can destroy every one, thus devastating the crop. In natural populations, on the other hand, genetic variation among individuals insures that some will enjoy protection against the agent of disease and part of the crop will survive. Since next year’s plants are offspring of these immune survivors, populations with abundant variability maintain a natural mechanism to purge themselves of disease.
The Irish, growing their potatoes from a uniform stock, lost their entire crop in 1845. The same story can be told for most agricultural mainstays. Some scholars believe that the mysterious collapse of classic Maya civilization was precipitated by a virus, borne by leaf hoppers dispersed on high-altitude air currents, that wiped out their corn crop virtually overnight. Corn continues to plague us with similar problems. During the summer of 1970, a new mutant strain of Southern Leaf Blight Fungus swept across American cornfields at rates of fifty miles or more a day, devastating all plants bred to contain a genetic element called Texas cytoplasmic male sterility factor.
To avoid this dilemma, breeders try to beef up genetic variability by hybridizing their successful but uniform stocks with different strains. For corn, a major source of potential hybridization lies in a plant of markedly different appearance, the New World grass known as teosinte. For example,
Zea diploperennis
, a recently discovered species of teosinte, is the only known source of immunity to three of the major viruses that afflict domestic corn. (This species is also a perennial rather than an annual like corn, thus giving potential substance to an old dream that, by hybridization, breeders might produce a perennial corn that survives from season to season and need not be replanted from seed each year.)
It may seem strange at first that a plant so different in appearance from corn should be sufficiently similar in genetic structure to permit hybridization. True, young plants of corn and teosinte are indistinguishable, but after they flower, the differences in adult structures could hardly be more profound. The business end of corn is a large cob bearing numerous rows of kernels (the technical term, polystichous—simply meaning many rowed—has a lovely ring). The cob and kernels are female, and they reside at the
terminal
end of stout branches
lateral
to the main stem (mark this well, for these positions become crucial in my developing argument). Many people don’t recognize this position because corn ears just seem to be stuck to the sides of the main stem. But the husks that so completely enclose the ear are actually remnants of leaves that originally formed on a longer lateral branch. They cover the cob, which is, indeed, terminal on a drastically shortened lateral branch. The central stem bears a male tassel, the source of pollen, at its terminal end. Thus, corn grows separate male and female structures: the tassel, terminal on the main stem, is male; the ears, terminal on lateral branches, are female.
Teosinte, on the other hand, grows a central stem and many long lateral branches of comparable length and strength. Each branch ends in a male tassel. The female ears, quite unlike corn, grow laterally, not terminally, from the lateral branches. The teosinte “ear” is also a miserable analog or runt compared with the majestic ear of corn. It contains (depending on the race of teosinte) six to twelve triangular kernels in two rows (technically distichous) telescoped into one because the triangular ends of the opposing kernels interdigitate. The kernels are surrounded by a stony outer covering and are quite useless as human food unless popped (as in popcorn) or laboriously ground and separated from their inedible covering. (Corn kernels are soft and naked, immediately available for food because their covering structures are not only pliant, but so reduced in size that they surround only the base of the kernel.)
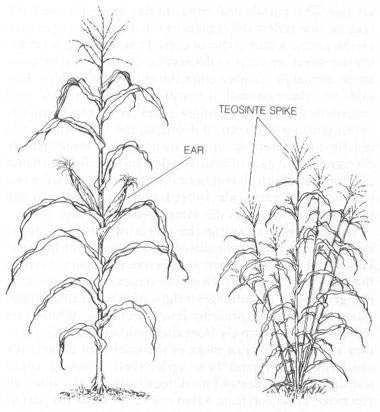
In modern corn (left), female ears are terminal on lateral branches; in teosinte (right), male tassels are terminal on lateral branches, while female ears are lateral on the lateral branches. Thus, the modern corn ear is the homolog of a teosinte tassel spike. See text for explanation.
REPRINTED FROM NATURAL HISTORY
.
Yet, despite these differences, corn and teosinte hybridize without any impediment, producing cobs of intermediate size. This paradoxical compatibility exists for two basic reasons that reflect the subject of this essay—a disquisition on the ancestry and origin of corn. First, teosinte is probably the direct ancestor of domestic corn (some experts disagree, although no one denies the close relationship). Second, no chromosomal disparities or even simple and consistent differences in single genes have been found between teosinte and corn. (Of course, the two forms could not be so different in appearance without some genetic divergence, but ease of hybridization and our failure to find differences indicate that genetic distinction between the two forms must be minuscule. Indeed, botanists place corn and the annual teosintes in the same species,
Zea mays
.)
The teosinte theory for the origin of corn has always suffered from one major dilemma: How could it happen? How could the teosinte ear, so different from corn, become the modern cob? Corn, like all our major domestic cereals, is a grass. The evolutionary origin of other major grains, wheat for example, presents fewer problems. Wheat ears differ only quantitatively from their wild grassy ancestor—they are essentially just more of the same, but bigger. We can easily understand how agricultural selection could translate a wild ancestor into domestic wheat. But how can you make a corncob from a teosinte ear or from any part of teosinte? They are constructed so differently.
In the standard version of the teosinte hypothesis—which I will reject here in favor of a radical alternative—the teosinte ear is, nonetheless, gradually transformed into a modern corn ear. It adds rows gradually, while the hard outer covering softens and retracts from the kernels. This scenario seems so obvious and so consistent with our usual view of evolutionary transformation. The tradition of gradual change from teosinte ear to corn ear dates at least to Luther Burbank, the great “wizard” of early twentieth-century plant breeding, who claimed that he had transformed teosinte to corn in eighteen generations of selection. He was wrong. He had started, not with teosinte, as he thought, but with a corn-teosinte hybrid—and his selection had merely segregated and accumulated the genetic factors for corn. But his general argument for a gradual transformation of the teosinte ear into a corncob persisted. In a
Scientific American
article of January 1980, George Beadle, one of the great corn scientists of our age, proclaimed that “the cobs can be placed in an evolutionary continuum from teosinte to modern corn on the basis of progressive modifications.”
But this theory of gradual derivation from the teosinte ear encounters three great problems, perhaps fatal. First, corn appears suddenly in the archeological record about 7,000 years ago. The earliest ears, to be sure, are not as fat or as many-rowed as a modern cob, but they clearly represent corn, not something in between corn and teosinte. Second, as stated before, breeders have found no consistent genetic difference between corn and teosinte. If corn were the product of long and slow selection from teosinte, a considerable number of genetic changes should have accumulated. Both these arguments are negative and therefore not conclusive. Perhaps sudden appearance merely records our failure to find intermediates; perhaps the absence of genetic difference only means that we haven’t looked at the right parts of the right chromosomes.
The third argument is positive and more troubling for the hypothesis that corn ears arose from teosinte ears. Remember the point I asked you to flag some paragraphs back: the positions of teosinte and corn ears are not equivalent. The teosinte ear sprouts laterally from lateral branches; the corn ear grows terminally on lateral branches. In teosinte, the terminal structure on the main lateral branches is a male tassel, not a female ear. Therefore, by position—and I shall say in a moment why position is so important a criterion—the modern female ear of corn is equivalent to (or, as we say in technical parlance, is the homolog of) a male tassel spike.
This homology of male tassel spike to female ear has long been recognized (and stated) by many corn experts, but no one has previously exploited this fact to develop a hypothesis for the origin of corn. The obvious theory suggested by this homology may, at first, sound absurd, but it solves plausibly and with elegance all the classical problems of the teosinte hypothesis. In short, this new theory proposes that corn ears evolved rapidly from male tassel spikes by shortening of the lateral branches and suppression of teosinte ears below. Instead of a slow and continuous enlargement of female teosinte ears, we envision an abrupt transformation of male tassel spikes to small and primitive versions of a modern female corn ear.
Hugh H. Iltis, professor of botany and director of the Herbarium at the University of Wisconsin in Madison, developed this heterodox theory and recently published it in America’s leading professional journal (see bibliography).
*
I have no corn credentials and cannot make any proclamation about the truth or falsity of this intriguing idea. But I do want to illustrate its status as a plausible, potential example of an evolutionary process often dismissed with ridicule for want of understanding—the so-called hopeful monster.
We call parts of two organisms “homologous” when they represent the same structure by a criterion of evolutionary descent from a common ancestor. No concept is more important in unraveling the pathways of evolution, for homologies record genealogy, and false conclusions about homology invariably lead to incorrect evolutionary trees.
Homologous structures need not look alike. Indeed, the standard examples invoke organs quite dissimilar in form and function, for these “classics” are chosen to illustrate the idea that mere resemblance does not qualify as a criterion. Examples include the homology of the hammer and anvil bones of the mammalian middle ear with the jaw articulation bones of reptiles, and the lung of land vertebrates with the air bladder of bony fishes.
How then can we recognize homology and thereby reconstruct the pathways of evolution? This most difficult question in evolutionary theory has no definite answer. No single criterion works in every case; all rules have well-known exceptions. We must evaluate proposed homologies by all available standards and accept or reject a hypothesis by the joint and independent affirmation of several criteria. Similarity in early embryology often works well for structures that become very different in adults: early mammalian embryos first develop their ear bones at the ends of their jaws—and this fact harmonizes with a well-established fossil sequence showing continual decrease of these two jaw bones and their eventual movement to the middle ear. But truly homologous organs may be modified by evolutionary changes in embryos that mask the pathways of descent.
A seemingly superficial detail—simple spatial relation with other parts—often serves well as a criterion of homology. As the old song goes, the foot bone truly is connected to the ankle bone, and such fundamental relationships are not easily altered in evolution. Thus, the so-called “positional criterion” of homology is probably the most respected and most often utilized of all standards. And by this criterion, modern female corn ears must have descended from teosinte male tassel spikes (for both features are alike in position at the terminal ends of lateral branches), and not from female teosinte ears.
