The Case for Mars (43 page)

FIGURE 9.2
Mars regolith/atmosphere dynamics under conditions of T¿=20 with a volatile inventory of 500 mb of CO
2
.
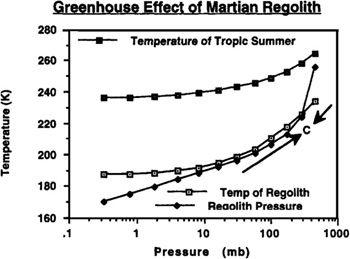
It can be seen in
Figure 9.2
that the atmosphere/regolith system under the chosen assumption of the temperature of desorption (T
d
) equaling 20°K has only one equilibrium point (where the two curves cross) that is stable. Once the polar cap is
gone, Mars’ global temperature and pressure will converge to this point. Thus, by the time the process is brought to a halt by the exhaustion of carbon dioxide reserves in the regolith and at the pole, an atmosphere with a total pressure of about 300 mbar, or 4.4 pounds per square inch, can be brought into being. Also shown in
Figure 9.2
is the day-night average temperature that will result in Mars’ tropical regions (T
max
) during summertime as the atmosphere thickens. Note that the curve approaches the 273°K freezing point of water, or, in terms of what interests us for terraforming, the melting point of water ice. With the addition of modest ongoing artificial greenhouse efforts, water ice and permafrost will begin to melt.
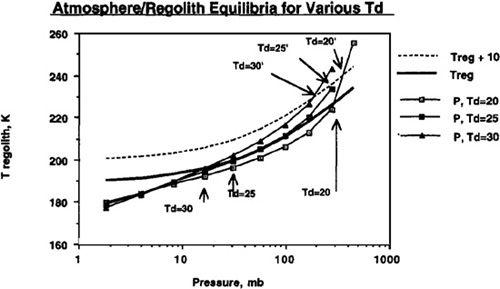
Assuming the temperature of desorption (T
d
) is 20°K may be optimistic, however, and the location of the equilibrium convergence point (point C in
Figure 9.2
) is very sensitive to the value we choose. In
Figure 9.3
we showhappens if we instead assume values of 25° Kelvin and 30° Kelvin for the temperature required to drive carbon dioxide out of the regolith. In these cases, the convergence point shifts fairly drastically from 300 millibar when assuming T
d
equals 20° Kelvin to 31 millibar when assuming T
d
equals 25° Kelvin, and 16 millibar when assuming T
d
equals 30° Kelvin. Such extraordinary sensitivity of the final condition to the unknown value of T
d
may appear at first to put the entire viability of the terraforming concept at risk. However, in
Figure 9.3
we also show (dotted line) what happens if we use artificial greenhouse methods to maintain the regolith temperature (T
reg
) 10° Kelvin above those produced by the carbon dioxide out-gassing itself. As mentioned previously, this could be done by pumping factory-produced CFCs into the atmosphere. You’ll see that this drastically improves the final global temperature and atmospheric pressure values when assuming the temperature of desorption (T
d
) to be 25° Kelvin or 30° Kelvin. In addition, we see that all three cases (T
d
equals 20° Kelvin, 25° Kelvin, or 30° Kelvin) converge upon final states with Mars possessing atmospheres with several hundred millibars pressure.
There’s one further unknown in the model that we should investigate, though it’s not quite as unknown as the temperature of desorption. That is the actual quantity of available carbon dioxide reserves we could find on Mars. The more reserves, the more carbon dioxide we can drive out of the regolith, and, hence, the thicker the atmosphere we can create. So we need to ask the questions, Is Mars “rich” or “poor” in carbon dioxide reserves, and How does the answer play out in our model? At the moment, the best we can do is assume either condition, rich or poor, and run it through our model to see what happens.
FIGURE 9.3
An induced 10°K rise in regolith temperature can counter effect of T
d
variations. Data shown assume a planetary volatile inventory of 500 mb CO
2
.
To understand just how the abundance of carbon dioxide could affect our terraforming efforts, and how the value of T
d
would interact with the quantities of carbon dioxide available, refer to Figures 9.4, 9.5, 9.6, and 9.7. In these figures, we can see the final atmospheric pressure and maximum seasonal average temperature equilibrium points in the Martian tropics based on assumptions of a “poor” Mars, possessing a total supply of about 500 millibar of carbon dioxide (50 mbar of carbon dioxide in the polar cap and 444 millibar in the regolith), or a “rich” Mars, possessing about 1,000 millibar of carbon dioxide (100 millibar in the polar cap and 894 millibar in the regolith). Recall that boosting the temperature of the regolith via artificial greenhousing methods made a significant difference in the final state of the atmosphere under differing values for the temperature of desorption. The same holds here, where different curves are shown under the assumptions that either no sustained artificial green
house effort is mounted after the initial polar cap release, or that continued efforts are employed to maintain the planet’s mean temperature at a level 5°, 10°, or 20° Kelvin above the value produced by the carbon dioxide atmosphere alone. For instance, you’ll see in
Figure 9.5
that even under an assumption of 40° Kelvin for the temperature of desorption, artificially maintaining atmospheric temperature at 20° Kelvin results in an overall tall thure boost of more than 40° Kelvin. More important, though, we can see that if a sustained effort is mounted to maintain the planet’s mean temperature 20° Kelvin above what native carbon dioxide reserves would produce, then a tangible atmosphere and acceptable pressures can be produced even if the temperature of desorption has a pessimistic value of 40° Kelvin.
FIGURE 9.4
Equilibrium pressure reached on Mars with a planetary volatile inventory of 500 mb CO
2
after 50 mb polar cap has been evaporated. DT (ΔT in text) is artificially imposed sustained temperature rise
.
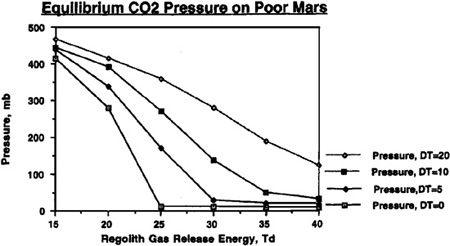
FIGURE 9.5
Equilibrium maximum seasonal (diurnal average) temperature reached on Mars with a planetary volatile inventory of 500 mb CO
2
after 50 mb polar cap has been evaporated
.
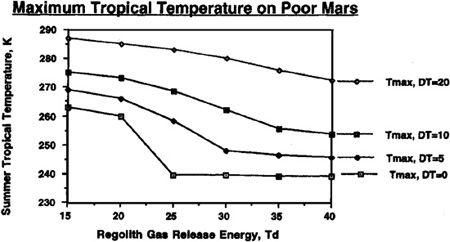
FIGURE 9.6
Equilibrium pressure reached on Mars with a planetary volatile inventory of 1,000 mb CO
2
after 100 mb polar cap has been evaporated
.
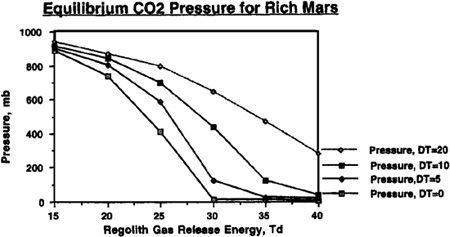
FIGURE 9.7
Equilibrium maximum seasonal temperature (diurnal average) reached on Mars
with
a planetary
volatile inventory
of 1,000 mb CO
2
after 100 mb polar cap has been evaporated
.
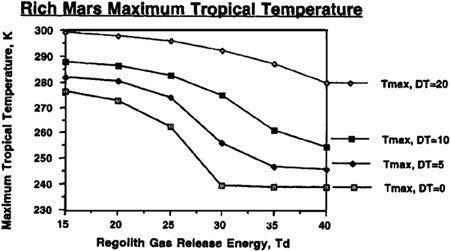
The important conclusion to be drawn from this analysis is that while the final conditions on a terraformed Mars may be highly sensitive to the currently unknown value of the energy required to libera
te carbon dioxide from the regolith, T
d
, they are even more sensitive to the level of sustained artificially induced greenhousing. Put simply, the final conditions of the atmosphere/regolith system on a terraformed Mars are
controllable
. Efforts to raise the mean temperature of the planet above that produced solely by releasing native reserves of carbon dioxide can overcome constraints imposed by even extreme values for T
d
.
HOW FAST WOULD THE ATMOSPHERE COME OUT OF THE REGOLITH?
What we have looked at so far are the final conditions after all the carbon dioxide we can get has evaporated from the polar cap and been liberated from the regolith. The stuff obtained from the cap comes off quickly, but forcing out adsorbed carbon dioxide from regolith at significant depth could take some time. For terraforming to be of practical interest, the rate at which all this occurs is important. After all, if it takes 100 million years for a substantial amount of gas to come out of the regolith, the fact that it comes out eventually is rather academic.
