The Case for Mars (25 page)

Now, manned Mars missions are likely to cost billions of dollars each. It is true that this cost can be reduced by introducing technologies such as nuclear thermal propulsion or cheaper launch vehicles. However, while such efforts are to be heartily encouraged, it must be pointed out that introduction of any of these technologies will cost billions, and only reduce Mars mission costs by around a factor of two. The expansion of surface mobility, on the other hand, is likely to be cheaper, and can potentially increase missio
n exploration effectiveness a hundredfold or even more.
It is clear that there is nothing more important in determining the cost-effectiveness of a program of human Mars exploration than the degree of mobility provided on the surface of the planet.
MARS CARS
Options abound for building Mars cars. Wheels, treads, half-tracks, and even motorized legs are all viable choices for getting around. What matters most is the power that actually moves the vehicle.
The only cars used in space to date have been the Apollo Lunar rovers, which were unpressurized electric vehicles powered by batteries. If we employed the latest advanced lithium-ion batteries (such as are used in camcorders) and gave them enough charge to power the rover for ten hours, such a system could be madeo produce about 10 watts of power for every kilogram of its weight. If instead of using batteries we employed hydrogen/oxygen fuel cells such as those on the Space Shuttle to provide the electric power, the system power/mass ratio could be raised to about 50 W/kg. That’s certainly an improvement, but it pales against a much more familiar household technology.
Internal combustion engines can have power/mass ratios of 1,000 W/kg. That’s twenty times higher than that of a hydrogen/oxygen fuel cell, one hundred times that of the battery-driven system. A combustion engine delivers far more power with far less mass than anything else (that’s the main reason why they are preferred for the vast majority of vehicle applications on Earth), and that has great implications for our Mars cars. For a given life-support system mass, the vehicle’s range will be directly proportional to its speed, which is in turn proportional to the power. But if you try to match one of the competing option’s power level with that of the combustion engine’s, the competing option’s weight will rapidly become excessive. Imagine a rover equipped with 50 kW (about 65 hp) of power. The mass of the required internal combustion engine would only be about 50 kilograms, while a set of fuel cells delivering that much power would weigh in at 1,000 kilograms. The combustion-powered car could thus take along 950 kilograms of additional science equipme
nt and consumables compared to a fuel-cell-powered vehicle of equal power, and again have much greater endurance, capability, and range. Furthermore, the fact that the combustion-powered vehicle is virtually power unlimited allows sortie crews to undertake energy-intensive science at a distance from the base that would otherwise be impossible. For example, a combustion vehicle sortie crew could drive to a remote site and generate 50 kW to run a drilling rig to try to reach the Martian water table. Rover data transmission rates are also proportional to power, and can therefore also be much higher, which in turn increases both crew safety and sortie science return. Combustion engines can also be used to provide high power for either main base or remote site construction activity (bulldozers, etc.). The bottom line is that the greater power density of combustion-powered engines provides for greater mobility with much smaller, lighter, and far more capable vehicles, and that translates into a more potent and cost-effective Mars exploration program all-around. If you want to do anything serious on Mars, you will need to employ combustion-powered vehicles.
But there’s a hitch. The use of combustion-powered vehicles is very fuel-intensive. For example, I estimate that a 1-tonne pressurized ground rover would require about 0.5 kilograms of methane/oxygen bipropellant to travel one kilometer. Thus, an 800-kilometer round-trip excursion would consume about 400 kilograms of propellant. Traveling at an average rate of 100 kilometers a day, this would only represent an eight-day sortie. In the course of a 600-day surface stay, many such excursions would be desired to make effective use of the available time. If the rover is employed in this way for just 300 of the 600 days,
15 tonnes
of propellant will be used. Having to import that much mass from Earth just to support rover operations would be a logistics disaster. If you want to be able to take advantage of combustion-powered vehicles on Mars, you have to be able to make their propellant on Mars.
The type of combustion engine used in a Mars car could be any of the common cycles in use on Earth today, including internal combustion, diesel, or gas turbine. However, if you try to burn a pure rocket propellant combination, such as methane/oxygen in a combustion engine, the result will be an engine that burns too hot to allow for the type of reliability and long life we need in a car motor. Diluting the burning mixture with atmospheric carbon dioxide dr
awn in by a fan gets around this problem. The carbon dioxide acts as an inert buffer, bringing down the flame temperature in the same way that nitrogen in air does on Earth.
The range of a ground rover powered by chemical combustion will depend critically upon the energy/mass ratio of the propellant we use. While in principle any bipropellant combination could be used, transportation logistics dictate that at least most of the propellant used be manufactured on Mars out of indigenous materials. A list of potential combinations is given in
Table 6.2
.
The Martian atmosphere is 95 percent carbon dioxide, and thus the hydrogen/carbon dioxide (H
2
/CO
2
) and hydrazine/carbon dioxide (N
2
H
4
/CO
2
) combinations given in
Table 6.2
can function as air-breathing engines, much in the manner that internal combustion and jet engines do on Earth. In these cases, therefore, the energy/mass ratio given is that of the energy release per unit mass of the non-carbon dioxide fuel, since the carbon dioxide does not have to be carried by the vehicle. It can be seen that from the point of view of energy/mass ratio, the hydrogen/carbon dioxide engine is superior to all other options considered. However, storing hydrogen presents formidable problems that probably makes the use of such a system on a ground rover impractical. Taking that into consideration, the high energy density of methane/oxygen would appear to make it the preferred option. This is fortunate, because it turns out that methane/oxygen is the easiest propellant to make on Mars. It also is the best option to use as the propellant for rocket vehicles taking off from Mars. As we have seen, the Mars Direct plan uses methane/oxygen as the propellant for the ERV. Therefore, the same in-situ propellant plant (ISPP) that makes our rocket propellant also can be used to make our rover propellant.
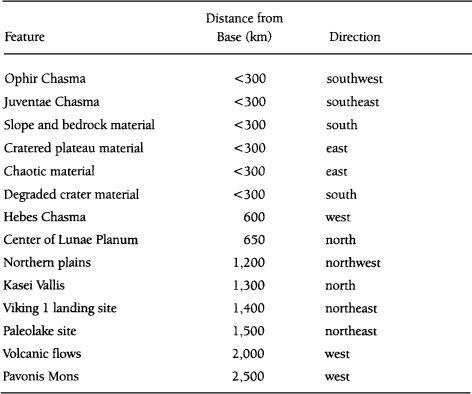
TABLE 6.2
Potential Bipropellants for Use in Mars Mobility Vehicles
So the rover will burn methane/oxygen diluted with carbon dioxide. The waste product of this engine will be carbon dioxid
e and water. The carbon dioxide is of no value—more can always be obtained from the Martian air—so it will be vented as exhaust. The water is another matter, however. Properly designed Mars cars will therefore carry condensers that will allow them to recapture the water portion of their engine combustion products. (This is not hard to do. U.S. Navy dirigibles operating in the 1920s did exactly the same thing. They needed their exhaust water for ballasting purposes.) At the conclusion of a given rover sortie, the condensed waste water will be brought back to the base to be combined with carbon dioxide and synthesized back into methane/oxygen propellant by the base’s chemical plant. If 90 percent of the water is recaptured, this system will allow the rovers to reuse the same propellant supply 10 times over.
How about rover life support? Well, using the same ISPP units responsible for propellant manufacture, unlimited quantities of oxygen can be readily produced on the Martian surface out of the carbon dioxide that comprises 95 percent of the Martian atmosphere. However, nitrogen and argon combined only compose about 4.3 percent of the Martian atmosphere, and thus buffer gas for breathing will be much harder to come by. (You can use carbon dioxide as a buffer gas for engines, but not for breathing. In concentrations above 1 percent it is toxic to humans.) It is therefore imperati that the habitats and pressurized vehicles operate at the lowest buffer gas partial pressures possible. For the surface habitat I recommend the 5 psi (3.5 lbs oxygen, 1.5 lbs nitrogen) atmosphere used by NASA astronauts in the long duration Skylab missions flown in the 1970s.
Apollo crews, however, operated on two-week missions in an atmosphere consisting of 5 psi oxygen and
no
buffer gas. Since the maximum rover excursion will be of this order, this is what I recommend for the pressurized rovers. There are major advantages to doing things this way. Such a low-pressure rover would require no
airlock, and so could be built much lighter than would otherwise be possible. When they want to leave the rover (“go EVA”), the astronauts inside would simply don their spacesuits, purge the pure oxygen atmosphere in the rover cabin, and then open the hatch and walk outside. Since no nitrogen is in the air mixture, this depressurization could be done very quickly; without nitrogen in your blood you can’t get the bends. Assuming a rover interior volume of 10 cubic meters, 3.3 kilos of oxygen would be lost each time the rover was depressurized in this way. If part of the rover’s interior atmosphere were pumped into a compressed oxygen cylinder prior to valving, oxygen losses would be reduced further, but in any case the losses could easily be made up by in-situ production of oxygen at the base. The low-pressure rover would allow the use of a low-pressure (3.8 psi oxygen, no buffer gas, as in Apollo) spacesuit for EVAs, with no pre-breathing period to prepare for the EVA required. Such a suit would be the lightest and most flexible possible, and thus enhance the quality of surface field science performed. (The current Shuttle spacesuits are virtually miniature spaceships, and are much too heavy to be used on Mars.) Since the oxygen is replaceable, a simple once-through system in which exhaled air is vented directly to the environment (in the manner of SCUBA gear) would be feasible, allowing a great simplification in spacesuit design. Such a simplification would not only further the goal of reduced spacesuit mass, but would dramatically enhance spacesuit serviceability, reusability, and reliability, making possible a Mars surface mission incorporating not tens, but
thousands
, of EVAs.
Assuming a breathing rate of 5 gallons a minute, each astronaut using such a low-pressure oxygen “scubasuit” would expend 1.3 kilograms of oxygen in the course of a four-hour EVA. Thus, if two astronauts were to perform two EVAs each per rover excursion day, venting the rover twice in the process, 12 kilograms of oxygen would be used up. If the rover were to be operated in this manner every day of the 600-day surface stay, a total of 7 tonnes of oxygen would be used up. Wasting this much oxygen would be a burden if it had to be transported from Earth. If it were produced on Mars, though, it would require only twenty-four days of operation of an ISPP plant driven by a 60 kWe reactor.
MAKING PROPELLANT ON MARS
It should be evident by this point in the discussion that both the ability to get to Mars affordably and to do anything meaningful once we get there depend critically upon one key technology: the in-situ production of propellant out of the Martian atmosphere. But is this really possible? Absolutely. In fact, all of the chemical processes used in the Mars Direct plan have been in large-scale use on Earth for over a century.
The first step in propellant production is to acquire the required raw materials. Since the hydrogen component of the bipropellant mixture represents only about 5 percent of the total propellant weight, it can be imported from Earth. Heavily insulating the hydrogen tanks with multilayer insulati Earn reduce in-space boiloff of liquid hydrogen to less than 1 percent per month during the six-to-eight-month interplanetary transit without any requirement for active refrigeration. Since the hydrogen raw material is not going to be directly fed into an engine, it can be gelled with a small amount of methane to prevent leaks. Gelling the hydrogen cargo will also reduce boiloff further (as much as 40 percent) by suppressing convection within the tank.
