Ross & Wilson Anatomy and Physiology in Health and Illness (179 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

Also called
osteogenesis
or
ossification
, this begins before birth and is not complete until about the 21st year of life (
Fig. 16.6
). Long, short and irregular bones develop in the fetus from rods of cartilage,
cartilage models
. Flat bones develop from
membrane models
and sesamoid bones from
tendon models
.
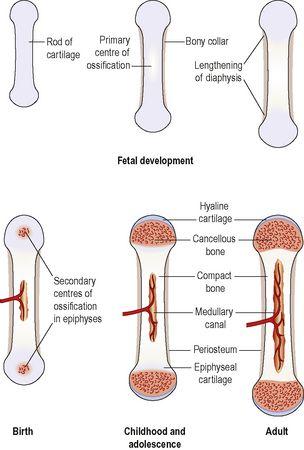
Figure 16.6
The stages of development of a long bone.
During the process of bone development, osteoblasts secrete osteoid, which gradually replaces the initial model; then this osteoid is progressively calcified, also by osteoblast action. As the bone grows, the osteoblasts become trapped in the matrix of their own making and become osteocytes.
Development of long bones
In long bones the focal points from which ossification begins are small areas of osteogenic cells, or
centres of ossification
in the cartilage model. This is accompanied by development of a bone collar at about 8 weeks of gestation. Later the blood supply develops and bone tissue replaces cartilage as osteoblasts secrete osteoid components in the shaft. The bone lengthens as ossification continues and spreads to the epiphyses. Around birth, secondary centres of ossification develop in the epiphyses, and the medullary canal forms when osteoclasts break down the central bone tissue in the middle of the shaft. During childhood, long bones continue to lengthen because the epiphyseal plate at each end of the bone, which is made of cartilage, continues to produce new cartilage on its diaphyseal surface (the surface facing the shaft of the bone,
Fig. 16.6
). This cartilage is then turned to bone. As long as cartilage production matches the rate of ossification, the bone continues to lengthen. At puberty, under the influence of sex hormones, the epiphyseal plate growth slows down, and is overtaken by bone deposition. Once the whole epiphyseal plate is turned to bone, no further lengthening of the bone is possible.
Hormonal regulation of bone growth
Hormones (see
Ch. 9
) that regulate the growth, size and shape of bones include the following.
•
Growth hormone
and the thyroid hormones,
thyroxine
and
tri-iodothyronine
, are especially important during infancy and childhood; deficient or excessive secretion of these results in abnormal development of the skeleton (
p. 221
).
•
Testosterone
and
oestrogens
influence the physical changes that occur at puberty and help maintain bone structure throughout life. Rising levels of these hormones are responsible for the growth spurt of puberty, but later stimulate closure of the epiphyseal plates (
Fig. 16.7
), so that bone growth lengthways stops (although bones can grow in thickness throughout life). Average adult male height is usually greater than female, because male puberty tends to occur at a later age than female puberty, giving a male child’s bones longer to keep growing. Oestrogens are responsible for the wider female pelvis that develops during puberty, and for maintaining bone mass in the adult female. Falling oestrogen levels after menopause can put postmenopausal women at higher risk of bone fracture (osteoporosis,
p. 424
).
•
Calcitonin
and
parathyroid hormone
(
p. 214
) control blood levels of calcium by regulating its uptake into and release from bone. Calcitonin increases calcium uptake into bone, and parathormone decreases it.
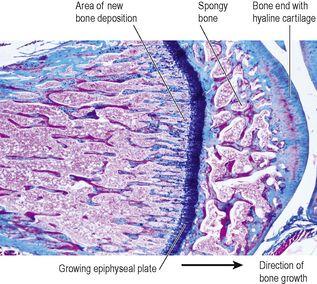
Figure 16.7
Light micrograph of the end of a growing bone, showing the epiphyseal plate.
Although the length and shape of bones does not normally change after ossification is complete, bone tissue is continually being remodelled and replaced when damaged. Osteoblasts continue to lay down osteoid and osteoclasts reabsorb it. The rate in different bones varies, e.g. the distal part of the femur is replaced gradually over a period of 5 to 6 months.
Exercise and bone
Although bone growth lengthways permanently ceases once the epiphyseal plates have ossified, thickening of bone is possible throughout life. This involves the laying down of new osteons at the periphery of the bone through the action of osteoblasts in the inner layer of the periosteum. Weight-bearing exercise stimulates thickening of bone, strengthening it and making it less liable to fracture. Lack of exercise reverses these changes, leading to lighter, weaker bones.
Diet and bone
Healthy bone tissue requires adequate dietary calcium and vitamins A, C and D. Calcium, and smaller amounts of other minerals such as phosphate, iron and manganese, is essential for adequate mineralisation of bone. Vitamin A is needed for osteoblast activity. Vitamin C is used in collagen synthesis, and vitamin D is required for calcium and phosphate absorption from the intestinal tract.
Bone markings
Most bones have rough surfaces, raised protuberances and ridges that give attachment to muscle tendons and ligaments. These are not included in the following descriptions of individual bones unless they are of particular note, but many are marked on illustrations. Bone markings and related terminology are defined in
Table 16.1
.
Table 16.1
Terminology related to the skeleton
| Term | Meaning |
|---|---|
| Articulating surface | The part of the bone that enters into the formation of a joint |
| Articulation | A joint between two or more bones |
| Bony sinus | A hollow cavity within a bone |
| Border | A ridge of bone separating two surfaces |
| Condyle | A smooth rounded projection of bone that forms part of a joint |
| Facet | A small, generally rather flat, articulating surface |
| Fissure or cleft | A narrow slit |
| Foramen (plural: foramina) | A hole in a structure |
| Fossa (plural: fossae) | A hollow or depression |
| Meatus | A tube-shaped cavity within a bone |
| Septum | A partition separating two cavities |
| Spine, spinous process or crest | A sharp ridge of bone |
| Styloid process | A sharp downward projection of bone that gives attachment to muscles and ligaments |
| Suture | An immovable joint, e.g. between the bones of the skull |
| Trochanter, tuberosity or tubercle | Roughened bony projections, usually for attachment of muscles or ligaments. The different names are used according to the size of the projection. Trochanters are the largest and tubercles the smallest |
Healing of bone
Bone fractures are classified as:
•
simple
: the bone ends do not protrude through the skin
•
compound
: the bone ends protrude through the skin
•
pathological
: fracture of a bone weakened by disease.
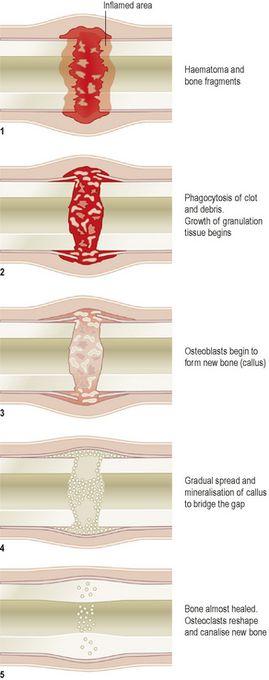
Following a fracture, the broken ends of bone are joined by the deposition of new bone. This occurs in several stages (
Fig. 16.8
).
1.
A haematoma (collection of clotted blood) forms between the ends of bone and in surrounding soft tissues.
2.
There follows development of acute inflammation and accumulation of inflammatory exudate, containing macrophages that phagocytose the haematoma and small fragments of bone without blood supply (this takes about 5 days). Fibroblasts migrate to the site; granulation tissue and new capillaries develop.
3.
New bone forms as large numbers of osteoblasts secrete spongy bone, which unites the broken ends, and is protected by an outer layer of bone and cartilage; these new deposits of bone and cartilage are called
callus
.
4.
Over the next few weeks, the callus matures, and the cartilage is gradually replaced with new bone.
5.
Reshaping of the bone continues and gradually the medullary canal is reopened through the callus (in weeks or months). In time the bone heals completely with the callus tissue completely replaced with mature compact bone. Often the bone is thicker and stronger at the repair site than originally, and a second fracture is more likely to occur at a different site.
Figure 16.8
Stages in bone healing.
Factors that delay healing of fractures
Tissue fragments between bone ends
Splinters of dead bone (
sequestrae
) and soft tissue fragments not removed by phagocytosis delay healing.
Deficient blood supply
This delays growth of granulation tissue and new blood vessels. Hypoxia also reduces the number of osteoblasts and increases the number of chondrocytes that develop from their common parent cells. This may lead to cartilaginous union of the fracture, which results in a weaker repair. The most vulnerable sites, because of their normally poor blood supply, are the neck of femur, the scaphoid and the shaft of tibia.
Poor alignment of bone ends
This may result in the formation of a large and irregular callus that heals slowly and often results in permanent disability.