Outbreak! Plagues That Changed History (11 page)
Read Outbreak! Plagues That Changed History Online
Authors: Bryn Barnard

Alexander Fleming was searching for a bacterial cause of the Spanish flu when he discovered the first antibiotic. A stray spore of a mold,
Penicillium notatum,
floated onto one of his petri dishes that was already infected with a culture of staphylococcus bacteria. Fleming noted that the mold produced a substance that inhibited the growth of the bacteria. He called it “penicillin.”
Fleming didn’t follow up on his discovery, but ten years later, two other British doctors, Howard Florey and Ernst Chain, tried to find out if penicillin had medical potential. When World War II created a huge need for drugs to treat wounded soldiers, these experiments were accelerated. At the time, soldiers were losing limbs and dying from even tiny wounds that festered and progressed to gangrene. Penicillin, though useless against viruses, stopped many bacterial infections with near-miraculous power. Soldiers who would have died from infection in previous wars could now return to the battlefield, giving the Allies a significant advantage over their enemies. Penicillin production was transferred to the United States and ramped up a millionfold, from petri dishes and lab trays to huge brewery vats. The strain of
Penicillium
changed, too, from the low-yield fungus in Fleming’s petri dish to a more productive strain discovered on a moldy cantaloupe in Peoria, Illinois. (A mutated version of that species,
Penicillium chrysogenum,
is still used today.) By 1945, the United States was producing enough penicillin to treat a quarter million patients a month. The age of antibiotics had begun.
“It is time to close the door on infectious disease.”
When United States surgeon general William Stewart made that pronouncement in 1967, it seemed neither hubris nor naïveté. It was a bold declaration in an age when anything seemed possible. At the time, smallpox was well on the way to being eliminated. Polio could be cured. Tuberculosis was in decline. Malaria and yellow fever were controlled. Measles was disappearing. Sexually transmitted diseases could be squelched. With infection nearly bested, chronic diseases like cancer and heart ailments seemed to be the focus of the future.
By century’s end, however, nearly all of those immunological gains had been reversed. Once-proud public health care systems were faltering, victims of their own success: as fear of infectious disease faded, many governments had slashed public health funding. Parents had begun to weigh the slightly possible adverse side effects of immunizations—from autism to death—against what they perceived as the dimming chance of disease. More and more people started opting out of vaccination programs. Diph-theria, pertussis, mumps, measles, and yellow fever reemerged as health problems. Meanwhile, bacterial infections that had been disappearing at midcentury not only had returned but had proved increasingly invulnerable to antibiotics. Worried infectious-disease professionals warned that unless something changed, even our most powerful antibiotics would eventually be useless.
As it turns out, only a single infectious genie—smallpox—has been successfully stuffed back in its bottle. By 1991, the disease disappointments of the late twentieth century would prompt weary Columbia University physician Harold Neu to this rueful wisdom: “Bacteria are cleverer than men.” By 2002, Flinders University microbiology professor Peter MacDonald would make an even more sweeping admission: “Germs are smarter than people and getting smarter all the time.”
Part of the problem is that since the days of Koch and Pasteur, we’ve thought of our relationship with disease as a kind of war. In the bloody twentieth century, where war was ubiquitous, disease was yet another enemy. Military terms began to define illness. Today the metaphors are pervasive: the human body is a fortress; microbes are the enemy; medicine is our weapon. In a time when everyone “fights” a cold, it’s hard to think of infectious disease any other way. The problem with this kind of thinking is that it suggests that in disease, as in war, we can beat microbes and they’ll stay beaten. Problem is, no one told the microbes.
Our relationship with pathogens is a never-ending competition. As we develop new measures to keep pathogens at bay, they evolve countermeasures to outwit us. So far, germs have proven smarter.
In hindsight, our current dilemma could have been predicted. As we have seen before, antibiotics don’t kill off all microbes, just the weak ones. A few resistant microbes survive, reproduce, and become dominant.
This is evolution, the force that is the real “invisible hand” pushing variation among and between all living things. Human beings, like microbes, have been pressured to change by evolution. We have evolved skin to keep microbes out, an elaborate internal immune system to neutralize those that get in, and sexual reproduction to shuffle our descendants’ genetic makeup, rendering them less vulnerable hosts. For both people and microbes, the process of change occurs over thousands of generations. The difference is that microbes reproduce in hours, not years. In an evolutionary race, they always win.
To improve our odds, we’ve added antibacterials, antivirals, antibiotics, and other medicines to our evolutionary armamentarium. But as early as 1946, only three years after the first use of penicillin, staphylococcus bacteria began showing resistance. With the mass deployment of the drug worldwide to treat a variety of infections, resistance increased. By 1952, three-fifths of all staph infections were resistant to penicillin. Today the figure is 95 percent.
In October 1943, a second antibiotic was discovered. It was called streptomycin, and it proved to be a total cure for tuberculosis. Soon the new drug was being sold by the ton around the world; by 1955, microbes began showing resistance. With the initial success of penicillin and streptomycin, drug companies began testing soil samples from all over the world to find new antibiotic-producing bacteria and fungi to replace them. Ultimately some eight thousand antibiotics were described, a fraction of which were safe enough to be used on people. Once a commercially useful antibiotic was isolated, it was mass-produced and deployed.
In every case, bacteria adapted and became resistant. Methicillin was deployed in 1960 to treat penicillin-resistant infections. Resistance showed up the following year. Vancomycin, a powerful, expensive antibiotic, was first deployed in 1956, and by the 1960s it was being used to treat methicillin-resistant staph. By 1986, some microbes actually thrived in the poison. Resistance to vancomycin’s replacement, linezolid, started showing up in 1999.
Today more than a hundred thousand tons of antibiotics are produced worldwide with annual sales of almost $5 billion. With so many pharmaceuticals flooding the environment, antibiotics have become a global evolutionary force. Hospitals, where bacteria and viruses have multiple opportunities to swap useful traits, have become centers of bacterial resistance and disease amplification. Each year millions of patients contract
nosocomial
(hospital-acquired) infections. Tens of thousands die. This is true in industrialized countries like the United States, where budget cuts have compromised basic hygiene procedures like sterilizing hospital laundry and isolating infectious individuals. It is also the case in Russia and Africa, where hospital poverty means vaccination syringes have to be reused hundreds of times, in effect injecting diseases from one patient into many others.
Bad as this is, it gets worse: some scientists are
deliberately
accelerating microbial evolution in novel directions. Around the world, military bioweapons laboratories are trying to create new pathogens with the characteristics of several different microbes (imagine plague, smallpox, and cholera deployed in a single organism). These perverse “chimeras” are engineered to overwhelm both human resistance and antibiotics. Released into the environment, either accidentally or on purpose, such pathogens could kill us all.
For a graphic reminder of what the world might be like were we to return to an age without modern hygiene and antibiotics, we have the example of the horrific Asian tsunami that occurred on December 26, 2004. In a few moments, a series of huge earthquake-generated waves killed over 150,000 people living around the Indian Ocean rim. In the days, weeks, and months that followed the disaster, thousands of injured survivors had no access to simple first aid: clean water, soap, bandages, and antibiotics. Simple wounds that could usually be treated with a twenty-five-cent pill became gangrenous. With no alternative, doctors had to do as they did during the American Civil War: cut off the dying limb to save the patient. In Indonesia, a month after the tsunami, doctors were performing so many amputations they started to run out of usable surgical saws.
Viruses can evolve even more quickly than bacteria. The story of HIV, the human immunodeficiency virus, is well known. HIV is the virus that causes acquired immune deficiency syndrome (AIDS).
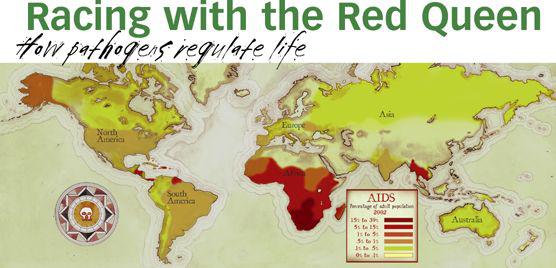
AIDS destroys the body’s immune system, opening the door for infection by other parasites, like tuberculosis (AIDS has been instrumental in accelerating the current TB epidemic). Currently about forty million people are infected with the virus. About four million new cases occur each year, and the rate is accelerating. Since 1981, when AIDS was identified, twenty million people have died of the disease.
HIV probably jumped to us from primates, most likely in Africa, where it is now a leading cause of death. The disease is transmitted from person to person by the exchange of bodily fluids. HIV evolves so rapidly that one person may host thousands of different HIV variants. Resistance to a single drug treatment evolves not in years or months but in days. There’s no cure, but HIV evolution can be temporarily arrested with an arduous, unpleasant “triple-drug cocktail.” It has to be taken several times a day, every day, for the rest of an infected person’s life. At current prices, it costs over $18,000 per patient per year. Recently, a variant of the virus was discovered that is immune even to this extreme treatment.