Life on a Young Planet (9 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

In general, then, stromatolites provide a sedimentary proxy record of microbial communities, revealing, like Friday’s footprints in the sand, the presence but not the character of their makers. Still, this information is helpful, for it shows that 600–800 million years ago, microorganisms colonized nearly every available surface on the Spitsbergen seafloor, from tidal flats to the open ocean.
Like stromatolites, organic matter is much more widely distributed than microfossils in Akademikerbreen rocks. Roger Summons, an Australian geochemist now at the Massachusetts Institute of Technology, has shown that the organic matter in Proterozoic sedimentary beds includes
biomarkers
, biological molecules of known origin that are preserved in and can be extracted from the rock. These molecules consist mainly of lipids that have survived a gauntlet of bacterial decay. (Sadly, nitrogen- and phosphorus-rich molecules like DNA have a vanishingly small likelihood of preservation in very old rocks.) To date, the search for biomarkers in Spitsbergen rocks has not been notably successful, but elsewhere, especially in shales of similar age exposed deep within the Grand Canyon, abundant and diverse biomarker molecules preserve molecular signatures of archaeans, bacteria, protozoans, and algae, most of which have left no recognizable microfossils.
Biology is encrypted in the Spitsbergen rocks in one additional, even more generalized way. Individual microorganisms are tiny, but their collective physiological effect can be strong enough to influence the chemical composition of the ocean. As a prime example, photosynthetic organisms influence the
isotopic
composition of carbonate minerals and organic matter deposited beneath the sea.
Isotopes provide our second set of Jacob Marley facts (the metabolic diversity of bacteria was the first). We have to come to grips with this bit of chemistry, because isotopes will allow us to track aspects of metabolic evolution through time. Moreover, as we’ll see in subsequent chapters,
isotopes provide a key to understanding the interplay between life and environmental change through our planet’s history.
Carbon atoms come in three varieties, distinguished by molecular weight. About 99 percent of all carbon is in the form of
12
C, meaning that it contains six protons and six neutrons, for a total molecular weight of twelve. (Electrons have negligible mass.) Most of the remaining 1 percent consists of
13
C, its extra neutron contributing to a molecular weight of thirteen. There is also a bit of
14
C (two extra neutrons), but this form is radioactive and decays to nitrogen on a timescale of millennia. Thus,
14
C doesn’t figure in discussions of very old rocks.
Because of their differing molecular weights, these
isotopes
behave differently in some chemical reactions. Notably, when photosynthetic organisms take up carbon dioxide to form organic molecules, CO
2
containing the lighter variety,
12
C, is incorporated more readily than CO
2
that contains
13
C. In consequence, the ratio of
13
C to
12
C in organic matter made by photosynthesis will be distinctly different from that of carbonate minerals formed in the same environment, a quantitative difference known as
fractionation
(
figure 3.4
). The difference is not large—about 25 to 30 parts per thousand—but it can easily be detected by geochemists armed with mass spectrometers. And this fractionation is preserved in sediments, providing us with a geochemical probe for ancient photosynthesis. (Organisms like ourselves that eat plants, algae, cyanobacteria, or other photosynthetic bacteria do not impart much additional fractionation in the process.) In the Spitsbergen rocks, the ratios of carbon isotopes in carbonates and organic matter consistently differ by about 28 parts per thousand—so photosynthesis fueled ecosystems in late Proterozoic oceans, just as it does today.
Chemistry also provides a paleobiological probe for sulfate-reducing bacteria. As noted in
chapter 2
, sulfate reducers play a key role in completing the marine carbon cycle, using sulfate ions (SO
4
2
−) to respire organic molecules. The sulfate is converted to hydrogen sulfide (H
2
S) that may combine with iron to enter the sedimentary record as pyrite (FeS
2
)—the fool’s gold sold in rock shops. Biological sulfate reduction shows a chemical preference for
32
S (16 protons and 16 neutrons) over the heavier isotope
34
S (two extra neutrons), resulting in sedimentary pyrite that is enriched in
32
S relative to gypsum formed from the same water body. Spitsbergen rocks indicate that the essential biological
components of the sulfur cycle, like those of the carbon cycle, were in place when the gray rocks of this arctic island accumulated.
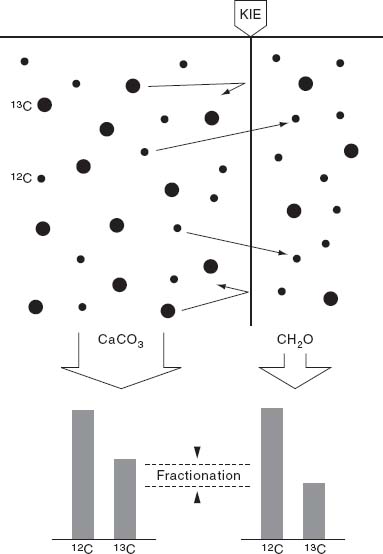
Figure 3.4.
Diagram illustrating how photosynthetic organisms fractionate carbon isotopes. Black dots on left side of the diagram depict carbon dioxide molecules that contain
12
C (smaller) or
13
C (larger). Photosynthetic organisms fix
12
CO
2
preferentially, with the result that the organic matter in photosynthetic organisms (and the organisms that eat them) is depleted in
13
C relative to its surroundings; biochemists speak of this as a kinetic isotope effect—hence, the label “KIE” in the figure. The isotopic
fractionation
imparted by organisms will be preserved in sediments as the difference in the ratio of
12
C to
13
C between limestone and organic matter in the same sample.
As observed on first sighting, the frozen Proterozoic rocks of Spitsbergen contain no bones, shells, or fossil trackways—nothing that would reward the casual collector (or Darwin!) on a weekend fossil hunt. But the apparent lack of fossils is deceptive—shells simply provide the wrong search image for Precambrian paleontology.
All of the carbonate minerals and organic carbon in the thick Spitsbergen succession bear the isotopic imprint of photosynthesis, and sulfur-containing minerals similarly preserve a metabolic signature of sulfate-reducing bacteria. Stromatolites document the ubiquity of microbial communities on the seafloor, while microfossils record aspects of biological diversity on the seafloor as well as in the water column.
When we look carefully, then, the fingerprints of biology are all over the Proterozoic rocks of Spitsbergen. The geological record
does
contain a record of early evolution that can be used to trim the Tree of Life. Our experience in Spitsbergen tells us how to approach ancient rocks and what to look for. But at 800 million years, the oldest beds on this desolate island are still relatively young. What happens when we apply these lessons to the bottom of the pile?
__________
1
Limestone consists of calcium carbonate (CaCO
3
) particles cemented together to form a rock; the closely related mineral dolomite [CaMg(CO
3
)
2
] also forms rocks, called dolomite or (especially in the British Isles) dolostone. Most dolomites seen in the geological record formed by the chemical alteration of sediments that were originally limy.
4 | The Earliest Glimmers of Life |
At 3.5 billion years old, sedimentary rocks of the Warrawoona Group, Western Australia, provide one of our earliest glimpses of life and environments on the young Earth. Warrawoona rocks contain stromatolites and microscopic structures interpreted as fossil bacteria, but these interpretations remain contentious. Chemical signatures provide more convincing evidence of life’s antiquity, although the type of biology they record is also uncertain. In geological investigations of Earth’s earliest life, we still look through a glass darkly.
J
AMES
H
UTTON
, the late-eighteenth-century father of geology, wrote in challenging prose, but he did manage one epigram familiar to every Earth scientist. The geological record, Hutton observed, shows “no vestige of a beginning, no prospect of an end.” Prospects of an end still seem remote, but over the past two decades, paleontologists have uncovered what can genuinely be regarded as vestiges of life’s beginnings.
On a sunny day in late July, I am off to North Pole to examine these vestiges. Heat and dust permeate the cab as our Land Rover rattles over the rutted dirt track. There are flies everywhere. This North Pole, you see, lies in northwestern Australia—its name, with characteristic Aussie humor, marking one of the hottest places on Earth (
figure 4.1
) Bumping along in the passenger seat, I listen idly to Frank Sinatra on the radio as I try to read the geological landscape around us. It isn’t easy. Trained to discriminate among shades of gray in arctic outcrops, my eyes fail me in the Australian bush, where everything is tinted red. Fortunately, I’m in good hands. At the wheel is Roger Buick, at the time a Harvard postdoctoral fellow and now professor of geology at the University of Washington. A brilliant iconoclast whose wiry frame and unruly mane camouflage the sophisticated scholar within, Roger has eyes of unparalleled geological keenness in these low rubbly hills.

Figure 4.1.
These low hills near North Pole, Australia, are built of sedimentary and volcanic rocks formed nearly 3.5 billion years ago. North Pole rocks preserve some of our earliest evidence of life and environments on the young Earth. Note Land Rover for scale.
Covered only sparsely by needle-sharp spinifex grass and scraggly acacias, the North Pole hills expose an extraordinary remnant of the early Earth, a thick succession of volcanic and sedimentary rocks called the Warrawoona Group that formed nearly 3.5 billion years ago. Folded and compressed between ovoid domes of granite, these rocks have for the most part been profoundly altered by metamorphic heat and pressure. Only at North Pole and a few other spots has some tectonic grace preserved them in a little-modified state. This is the place to ask questions about the antiquity of life.
Before asking such questions, however, we need to address an issue that was glossed over in our discussion of Spitsbergen rocks—proof of age.
How do we know that the North Pole rocks formed more than 3 billion years ago?
Geologic time can be measured in two ways. Any event discernible in the rock record can be used to divide Earth history into three intervals: the time preceding the event, the time of the event itself, and all subsequent time. By mapping the distribution and spatial relationships of rocks seen locally, a series of events can be placed in order of occurrence to form a
relative
timescale. In principle (though not always in practice), the rules are straightforward. Sediments, ash spewed from volcanoes, and lava flows settle by gravity onto the land surface or seafloor. For this reason, any such layered rock will be younger than the beds it covers. Volcanic rocks that intrude into other units must be younger than the rocks they pierce. And events that alter rock accumulations—folding, faulting, erosion, and metamorphism—obviously must postdate deposition.
