Life on a Young Planet (6 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

This exercise has been repeated numerous times, using dozens of gene families. Many trees yield the root position shown in
figure 2.2
, but others suggest different relationships among the three domains. No one tree satisfies all genetic data, forcing a startling conclusion. We think about genes being passed vertically through the tree from ancestor to descendant, but some genes must have been passed horizontally from one branch to another, perhaps by hopping a ride on a virus or by the uptake of DNA from dead cells. Contemporary organisms are, thus, genetic chimeras.
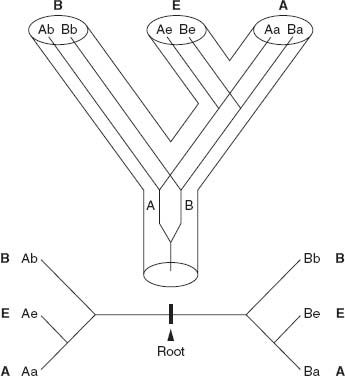
Figure 2.2.
Rooting the Tree of Life.
Top:
The genealogical relationships among Bacteria (B), Eucarya (E), and Archaea (A) are shown by the hollow cylindrical branches. Lines within the cylinders show the phylogeny of a gene that duplicated into forms A and B prior to the differentiation of the three domains from their last common ancestor.
Bottom:
The evolutionary relationships among the genes are shown here. Each half tree can be rooted relative to the other, allowing molecular biologists to reconstruct the genealogical relationships among eukaryotes, archaeans, and bacteria.
This revelation potentially casts doubt on the entire endeavor of constructing phylogenies from gene sequences, because gene trees and organism trees will not coincide when horizontal transfer has occurred. According to some biological Cassandras, the genes of microorganisms have been swapped so often and so promiscuously that no meaningful
tree of microbial
organisms
can be recovered from molecular comparisons. This possibility is both frustrating and tantalizing, but it may be overdrawn. Sorel Fitz-Gibbons of UCLA and Christopher House, now at Penn State, analyzed the distribution of all genes in the dozen or so organisms whose complete genomes had been sequenced by early 1999. The tree recovered by comparing universal gene distributions closely matches that inferred from ribosomal RNA gene sequences, suggesting that despite gene swapping, phylogenetic order underpins the genomes of bacteria and archaeans.
James Lake and Maria Riviera, also of UCLA, have even hypothesized that certain rules govern the likelihood of horizontal transfer.
Informational
genes that code for basic features of cell biology appear to be unlikely candidates for lateral exchange—ribosomal RNA genes fall into this category. In contrast,
operational
genes, genes or groups of genes that encode specific metabolic functions, may be passed from one lineage to another with relative ease by means of viruses or other vectors. For example, we know that bacterial tolerance to heavy metals can be gained by the uptake of particular genes.
Dawn is just breaking on the study of microbial genes and phylogeny, and as more and more genomes are sequenced in their entirety, new insights can be expected to topple current generalizations. For now, it is reasonable to view the Tree of Life as a reflection of microbial genealogy. But we must think of it as a genealogy of the microbial “chassis,” with specific features that “soup up” well-adapted organisms assembled, in part, by gene exchange across taxa.
The bacterial limb of the tree is profusely branched; at present we know of at least thirty major groups of bacteria, each more or less equivalent to the plant and animal kingdoms traditionally recognized by biologists. Most of the diverse metabolisms discussed in the previous section can be found on this limb. In particular, photosynthesis is a distinctly bacterial physiology. (How this squares with the obvious presence of photosynthesis in eukaryotic plants and algae is one of the great stories of evolutionary biology, but a tale best left for
chapter 8
.) Note, however, that photosynthetic lineages adorn only the upper branches of the bacterial limb. This suggests that Earth’s earliest ecosystems were fundamentally different from those that surround us today. Today, photosynthesis fuels biology
in most habitats. Early life, however, must have run on chemosynthesis. The earliest branches currently recognized on the bacterial limb contain chemosynthetic and heterotrophic organisms, many of which live at high temperatures in the absence or near absence of oxygen.
In contrast to Bacteria, the Archaea contain only two principal groups, albeit with hints of others yet to be characterized. One branch of archaeans is dominated by methanogenic organisms. Most bugs on this branch are obligate methane producers, but in at least three instances, individual lineages have evolved a more diverse metabolic repertoire that includes respiration. According to Gary Olsen of the University of Illinois, these “add-ons” are all encoded by genes transferred horizontally from bacteria. This emphasizes that horizontal transfer isn’t something that occurred once or twice in the earliest history of life; it is a continuing and persistent means of creating biological novelty.
Closely related to the methanogenic archaeans are the halobacteria, a distinctive group of microorganisms that gain energy from the sun, using a light-harvesting pigment strikingly similar to the rhodopsin in vertebrate eyes; halobacteria obtain the carbon they need to grow by absorbing organic molecules. The other major branch of the Archaea includes organisms that derive energy from chemical reactions between hydrogen and sulfur compounds.
Archaea are widely distributed across the Earth, but we still know relatively little about most of them. For example, only in 2001 was it discovered that tiny archaeans may be the most abundant organisms in many parts of the ocean; biologists have no idea how these microbes make their living. On the other hand, some of the best-characterized Archaea live in unusual places—
very
unusual places. The halobacteria, for example, thrive in waters that are ten times saltier than the ocean. (The striking magenta sheen of halobacteria can be seen from the air in commercial salt ponds, such as those that line the landing approach to San Francisco airport.) Other archaeans live in acid mine waste with a pH of 1. And the current world record holder for temperature tolerance is
Pyrolobus fumarii
, an archaean that can grow in deep-sea hydrothermal vents at 113ºC. (At the high pressures of the ocean bottom, water this hot remains liquid.) These
hyperthermophilic
organisms cannot grow at the temperatures used to pasteurize milk—not because they are too hot, but because they are too cold!
How do we think about such “extremophiles”? Are they merely fascinating oddballs, or do they tell us something fundamental about the history of life? Some, such as the halophiles, reside on distal branches of the Tree of Life, implying that these groups evolved relatively late in its history. In contrast, hyperthermophilic prokaryotes occupy a privileged position in the tree—they are found on the earliest branches of both the archaeal and bacterial limbs. This suggests that modern organisms are descended from ancestors that lived in hot environments. Thus, when we encounter microbial communities in the colorful hot springs of Yellowstone Park or in the midocean ridges that traverse the deep seafloor, we are glimpsing some of our earliest ancestors (color
plate 1
).
(Recently, geneticists have added an intriguing twist to this story. The amino acid sequences in proteins from living microorganisms can be used to reconstruct ancient proteins likely to have been present in the last common ancestor. Surprisingly, the putatively ancestral proteins synthesized from these reconstructions are not stable at high temperatures. If true, the last common ancestor of bacteria and archaeans could not have been hyperthermophilic at all. How to reconcile this finding with the Tree of Life remains a subject of debate. One possibility is that the earliest organisms evolved at moderate temperatures, but gave rise to (at least) two groups of descendants that colonized energy-rich hot springs. Now all we need is an exterminating angel to wipe out all life save the handful of lineages sheltered in hydrothermal foxholes. Giant meteor impacts would do nicely, and crater histories of the Moon and Mars indicate that early in its history (before 3.9 billion years ago), the inner solar system was pummeled again and again by gigantic meteors. Earth could not have escaped this drubbing. Indeed, Norman Sleep of Stanford University long ago proposed that life’s only refuge on the primitive Earth would have been hydrothermal vents in the deep seafloor. Thus, the deepest branches of the tree may tell us of both evolution and extinction when life was young.)
As implied earlier, the Tree of Life provides a road map for history of life, its branching order reflecting the successive radiations of biological diversity. The tree suggests that early ecosystems were centered on hydrothermal vent and spring systems, with the later appearance of photosynthesis enabling life to spread across the planet. Large, complex organisms
like plants and animals are evolutionary latecomers, confined to distal twigs on a eukaryotic branch formed mainly by microscopic organisms.
There is another way to interpret the tree. Because organisms in general, and microorganisms in particular, are commonly tied to specific habitats, the tree can be read as an environmental history of the Earth. For example, most early branching organisms do not use oxygen in metabolism, and many are killed by exposure to O
2
at even part-per-million levels. Organisms that can thrive when oxygen is present in moderate amounts branch later, and only at the tips of the tree do we find organisms like ourselves that require oxygen in high concentrations.
The Tree of Life, thus, makes predictions about Earth history that can be tested against the geological record. The first essential point of the tree is that the organisms and environments of our common experience are relatively recent features; the deep history of life is microbial. The other main point is that life has not evolved on a static planetary surface. Rather, life and environments have evolved together throughout our planet’s history, inexorably linked by the biogeochemical cycles in which both participate.
Armed with predictions from comparative biology, we can turn our attention back to the Cambrian Explosion, captured so vividly in the cliffs along the Kotuikan River. The Tree of Life supports Charles Darwin’s intuition that the Cambrian radiation of animals must have been preceded by a long antecedent history of life. Paleontologists wishing to reconstruct this history must focus on rocks deposited
before
the Cambrian period—on
Pre
cambrian rocks that document Earth’s early planetary development. We also need to replace zoological search images by pictures drawn from microbiology. But bacteria, archaeans, and simple eukaryotic microorganisms are tiny and fragile. Can we really expect them to have left an interpretable fossil record?
3 | Life’s Signature in Ancient Rocks |
Sedimentary rocks on the arctic island of Spitsbergen formed 600–800 million years ago, well before the Cambrian Explosion. These rocks contain no traces of animal life, but viewed under a microscope, they teem with tiny fossils of cyanobacteria, algae, and protozoans. More conspicuous are stromatolites, reeflike structures built by microbial communities. Most pervasive, however, are chemical signals imparted by microbial metabolisms. Such discoveries encourage us to explore much older beds for evidence of life’s earliest evolution.
S
PITSBERGEN
, an isolated and forbidding island halfway between Norway and the North Pole, is a starkly beautiful study in gray and white. The white is glacial ice that blankets most of the island; gray in various shades stripes the rocks that rise from the ice in great cliffs (
figure 3.1
). Tundra wildflowers add a splash of color, but vegetation is sparse. Century-old willows on coastal lowlands stand only a few inches above the ground, overtopped even by pale tufts of reindeer moss. Higher in the mountains only fairy rings of lichen, tiny but fiery orange, brighten the landscape. Seals and walruses sprawl languorously on grounded pack ice, and miniature reindeer graze on the miniature plants. Polar bears ply this coast, as well, fat from alfresco dinners of seal and curious about unusual sights like bright yellow tents pitched on a hill. Paleontologists sleep lightly in Spitsbergen.
In the mountainous northeastern quarter of the island, valley glaciers, majestic rivers of ice that flow with inexorable power but almost imperceptible speed, have cut deep incisions into the landscape—so deep, in fact, that the Empire State Building, placed on the ice, would barely peek above a valley rim. What am I doing here? I asked myself as I crawled across a cliff top, unable to walk upright in the late afternoon gale. Like the How did we get here? query in
chapter 1
, this question has a spectrum of possible answers. On that blustery afternoon in the Arctic, the question might well have been rephrased, Why don’t I work on tropical reefs? Transient disgruntlement aside, my question has a literal answer: my field partner, University of Iowa geologist Keene Swett, and I were logging a stratigraphic section through a thick succession of upper Proterozoic rocks exposed in the Spitsbergen cliffs. But once again, there is another answer, one that addresses why as well as what.
