Life on a Young Planet (34 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

Figure 11.7.
Expression of
Hox
genes along the body axis of a fruit fly (Insecta) and other arthropods, suggesting the molecular basis of morphological variation within the phylum. (Reprinted with permission from A. H. Knoll and S. B. Carroll, 1999. Early animal evolution: emerging views from comparative biology and geology.
Science
284: 2129–2137. Copyright 1999 American Association for the Advancement of Science)
We can now begin to understand how Cambrian animals could have evolved more rapidly than Darwin envisioned. Mutations in regulatory genes made rapid diversification possible.
If mutations in regulatory genes fueled Cambrian diversification, can we further infer that such mutations were more common in the Cambrian than at other times? I doubt it. Genes mutate in present-day animal populations and do so at rates that are probably similar to those of the Cambrian. Most of these mutations are lethal—they produce animals that don’t work. Some mutants survive in laboratory incubators, but we don’t seem to see them in nature. Animals that function poorly simply can’t compete in a world that is full of functionally sophisticated organisms.
This brings us to the crux of Cambrian evolution. To foment biological revolution, we need more than mutations; mutants must survive and reproduce, generating further variation on which natural selection can act. It is commonly assumed that evolutionary radiations begin with finely wrought innovations that take the world by storm. But this isn’t the case—innovations become honed by natural selection only with the passage of time. Biological radiations
begin
when a permissive ecology allows poorly functioning novelties to persist.
By “permissive,” I mean an ecological landscape in which competition for resources is rare or weak. (You don’t have to be good to win the game of evolution; you only have to be better than the other players.) Permissive ecologies may arise because environmental change makes new physiologies possible, or because an evolutionary novelty allows organisms to exploit resources in a new way, however poorly. Environmental catastrophe provides still another route—populations that survive mass extinction may radiate in the ecological emptiness that follows, as mammals did after the dinosaurs disappeared. As
we’ll see in
chapter 12
, latest Proterozoic and Cambrian history suggests that all three circumstances may have contributed to early animal diversification.
In 1996, Duke University biologist Greg Wray and his colleagues published a paper that set the paleontological world on its ear. Maybe fossils only preserve animals younger than 600 million years, they wrote, but the major bilaterian groups must have diverged from one another long before that, perhaps a billion years ago or even earlier. Wray’s conclusion didn’t come from geology, but rather from a particular reading of molecular biological data. “Molecular clock” estimates of evolutionary divergence times begin with the assumption that changes in the nucleotide sequences of genes accumulate in more-or-less clocklike fashion, with little variation through time or among taxa. If we accept this premise, we can measure the differences between genes in two species and use this to estimate when the lineages diverged from their last common ancestor. In fact, the starting assumption is contentious—many genes are known to violate it—but proponents of the molecular clock contend that violations can be recognized and eliminated from consideration.
Greg Wray’s team compiled nucleotide sequences for specific genes drawn from many different vertebrate animal species. Then, they calculated the differences in gene sequence between many different pairs of species and plotted these on a graph against the times when the species parted genealogical company, as estimated from fossils. (Vertebrates were favored because bones leave a pretty good fossil record.) Surprisingly (at least to me), the data for some genes fell along a more-or-less straight line (
figure 11.8
), suggesting that nucleotide changes had indeed accumulated in clocklike fashion.
So far, we’ve only compared known genetic differences versus known times of evolutionary divergence. The contentious part comes next, with the assumption that the rates of molecular change calibrated for vertebrates can be extrapolated to deeper branches of the animal tree. Making this assumption, Wray’s group measured gene sequence differences between pairs of protostome and deuterostome species and used these to estimate when the two great branches of bilaterian animals diverged from each other (
figure 11.8
). Genes for hemoglobin suggested a divergence time as early as 1.6 billion years, whereas a gene that codes for a
cytochrome oxidase enzyme implied more recent divergence—perhaps 800 million years ago—but still long before the first Ediacaran fossils.
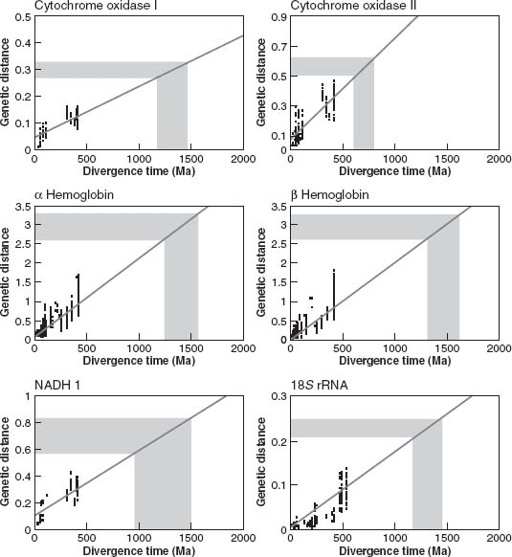
Figure 11.8.
The molecular clock, illustrated using data from Wray and colleagues (1996). Shaded area shows measured genetic distances between deuterostome and ecdysozoan or lophotrochozoan species. Projection onto
x
axis estimates time since divergence from the species’s last common ancestor. (Reprinted with permission from G. A. Wray, J. S. Levinton, and L. H. Shapiro, 1996. Molecular evidence for deep Precambrian divergences among metazoan phyla.
Science
274: 568–573. Copyright 1996 American Association for the Advancement of Science)
Stimulated by Wray’s provocative results, other labs have taken up the challenge of molecular clocks and animal origins. Published estimates for bilaterian divergence range from 1.6 billion to 650 million years ago, reflecting differences in the genes and computations used in different studies.
Many biologists are uncomfortable with the idea that rates of molecular change can be extrapolated from vertebrates to animals as a whole. Given this concern and the truly wild variation in age estimates, it is tempting to conclude only that molecular clocks keep poor time. This may make paleontologists feel better, but it glosses over a potentially important point. Whatever their inconsistencies,
all
molecular clock estimates published to date indicate that animals began to diversify much earlier than fossils suggest.
Recently, Kevin Peterson and Carter Takacs of Dartmouth College have approached the molecular clock from another angle, basing their calibrations on echinoderm genes and fossils rather than vertebrates. They estimate that the last common ancestor of mice and fruit flies lived 540–600 million years ago, in close agreement with the fossil record. Many paleontologists like this estimate because it doesn’t require an extended prehistory of the bilaterian supergroups (and suggests that we were right all along). But this doesn’t let paleontologists off the hook. Perhaps the split between protostome and deuterostome bilaterians occurred only 540–600 million years ago, but according to the Tree of Life, bilaterians and cnidarians must have parted company earlier—and the common ancestors of bilaterians and cnidarians must have split from sponges earlier still. Peterson and Takacs estimate that the early branches of the animal tree formed 700–750 million years ago. The oldest known sponge and cnidarians, however, are less than 600 million years old. Thus, even the conservative molecular clock estimates of Peterson and Takacs require up to 150 million years of animal history not yet recognized in rocks.
How do we reconcile molecular clocks that call for early animal divergence with a fossil record that identifies metazoans as evolutionary latecomers? Andrew Smith, an accomplished switch-hitter in molecular
phylogeny and paleontology, has outlined the options for reconciliation. There are only three.
Perhaps gene sequences diverged at unusually high rates early in animal evolution, undermining efforts to read time from molecules. The idea that genes might evolve rapidly during early diversification isn’t absurd or even ad hoc—evidence exists for rapid gene evolution during the explosive radiation of some younger groups. Thus, if gene sequences in Phanerozoic vertebrates diverged by, say, 1 percent every 50 million years, while genes in the earliest animals diverged by 1 percent in 10 million years, extrapolation of vertebrate rates would seriously overestimate the divergence times for early branches on the animal tree. That said, in cases where genes are known to have evolved rapidly early on and then slowed down, their change of pace has left a recognizable signal in the relative abundances of the four nucleotides that spell out gene messages. Smith scrutinized available data on animal gene divergence and found no evidence of evolutionary rate change.
Perhaps, Smith continued, genes are telling us the truth and paleontology has misled us. That is, maybe we haven’t sampled the geological record carefully enough and so missed animal fossils that sit in late Proterozoic rocks. Smith is modestly enthusiastic about this possibility, but having spent the better part of twenty years tromping around late Proterozoic rocks, I’m skeptical of the idea that by looking harder we’ll find Cambrian-like fossils in older beds. Trace fossils illustrate the point. It isn’t just that animal tracks and trails first appear in latest Proterozoic rocks, it is that they appear
everywhere
once they enter the record. In older rocks, on the other hand, you can search for a very long time without seeing anything.
6
Exceptional windows on the past like those of Doushantuo and Nama reinforce the idea that whatever we’ve over-looked
in late Proterozoic rocks, it isn’t large, complicated animals like those of the Cambrian.
As Smith points out, the third alternative is that molecular biology and paleontology are both right, but are telling us different things. This takes us back to the point made earlier that body plan evolution within a group is different from (and postdates) the genealogical divergence of a group from its relatives. Accurate or not, molecular clocks estimate times of evolutionary branching. Fossils document body plan evolution within animal phyla.
Given the phylogenetic inference that major eukaryotic groups diverged rapidly and fossil evidence that multicellular red algae existed more than a billion years ago, it isn’t crazy to conjecture that stem group animals emerged early as well. One simply has to accept that the earliest stem cnidarians and bilaterians were rare, gossamer, or minute organisms not likely to be preserved (or at least recognized) in the fossil record. But shared features of genetics and morphology place some limits on our speculation. Early cnidarians might have resembled modern
Hydra
, a tiny (up to one centimeter tall and rarely more than a millimeter wide) polyp that secretes no skeleton and leaves no mark on the sediment surface. Such animals would rarely if ever show up as fossils. In contrast, the last common ancestor of protostomes and deuterostomes was large and complicated enough to have left its calling card in sediments, at least if it moved across the seafloor or formed a preservable organic cuticle. Of course, those are big ifs—nematodes, for example, have probably been abundant throughout the Phanerozoic Eon, but they have left hardly any recognizable fossils (
figure 11.9
).
Accepting the qualitative if not necessarily the quantitative claims of molecular clock hypotheses also requires that we view Ediacaran events in a specific way—as the independent evolution of large size within major branches of animals that separated earlier. Why, we must ask, did large animals with their complicated body plans take shape so long after their tiny ancestors began to diversify?
At present, the problem remains unresolved. Paleontological tests of molecular clock hypotheses will require that we scour late Proterozoic sedimentary rocks in search of tiny but distinctive phosphatized fossils like those in Doushantuo rocks. Many are looking; as yet, no one has found anything convincing. But we also need to think hard about environmental events that might have stimulated animal evolution, or more accurately, the evolution of large, preservable animals, 600–580 million years ago. Moreover, recalling fossils introduced in earlier chapters, we must explain the
simultaneous
emergence of diverse seaweeds and planktonic algae, large protozoans
and
large animals at this time. We need to look carefully at the momentous physical upheaveals that shook the late Proterozoic world.

