Alien Universe (27 page)
Authors: Don Lincoln

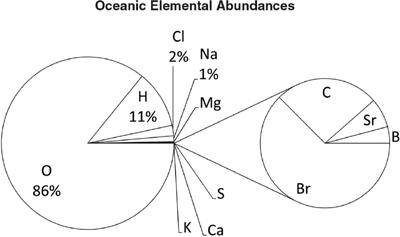
FIGURE 6.9
.
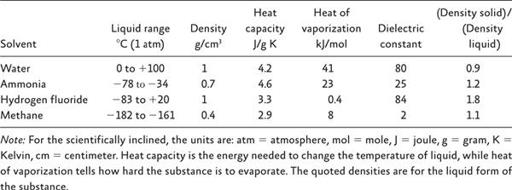
The elemental makeup of ocean water is a product of its chemical composition of water (H
2
O) but also the fact that it contains salt (NaCl). Carbon (C) is a trace component of seawater.
As a final look at elemental availability, we turn to the human body. While the whole point of this discussion is to see what elements are available as building blocks of life, it is natural to ask “yeah, but what elements actually form life?” This is shown (for humans only) in
figure 6.10
.
Carbon, oxygen, hydrogen, and nitrogen dominate human chemistry, with a handful of other elements joining the mix. Our blood reflects our origins in the Earth’s oceans. Calcium is used for bones and cellular metabolism. Trace minerals are found in our foods.
The fundamental question is whether other chemical compositions are possible for Aliens, and the answer must be yes. Biologists are still working out whether the composition of life on Earth is a historical accident or an inevitable consequence of the atomic properties of the elements and their relative abundances. Therefore, it is not at all surprising that astrobiologists haven’t worked out what form Aliens or even the less restrictive alien life must take. But the limitations of chemistry and elemental availability are surely important considerations for their discussions. The topics we have discussed here—from the number of atomic bonds, to bond strengths, to elemental availability and evolutionary accidents and pressures—resulted in us. While oxygen breathing, carbon-based life-forms are not inevitable, we now see the advantages of that particular recipe.
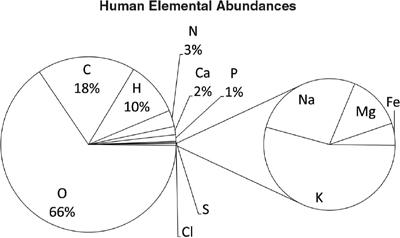
FIGURE 6.10
.
This plot shows the elemental abundances in the human body. We see why the crystal aliens in the
Star Trek: The Next Generation
episode “Home Soil” refer to humans as “ugly bags of mostly water.” Given the chemical abundances of the Earth’s crust and ocean, it is striking to see which elements are most present in living human tissue, with 97% coming from oxygen (O), carbon (C), hydrogen (H), and nitrogen (N).
Liquid Advantage
Life on Earth is universally water based, specifically liquid water. This naturally leads to two questions: Why liquid, and why water? The liquid question is the easier to answer. Matter typically exists in solid, liquid, and gaseous phases. The problem with the solid phase is the low mobility of chemicals. While solid-phase chemical mixing is possible, it is very slow. Life might form under those circumstances, but such life will never be an Alien in the way we mean it here. (Although we do need to keep in mind the idea of robotic life, as mentioned at the end of the chapter.) Further, unless the environment is totally dry, the advantages of liquid-based life are so manifest that either independently developed liquid-based life will out-compete the solid-based one or evolution will find a way for the solid-based life to adapt to using liquids.
In contrast, the gaseous phase of matter is supremely mobile. In fact, in many elementary school textbooks, a gas is defined as the phase of matter that fills up any volume into which it is introduced. So getting the gas molecules to move around isn’t a problem. What’s a problem is that a gas doesn’t do a good
job dissolving anything. While salt water can carry a goodly load of sodium and chlorine atoms, salt air only carries a little water, which itself contains the salt. So it is similarly unlikely that we will find life-forms (and especially Aliens) with a gaseous solvent.
So this leaves liquid. Liquid can move easily and is able to dissolve substances in it to move them around, like the salt in salt water. In order for a liquid to be a useful solvent, it must have two properties. First, to be useful a liquid must stay liquid under many conditions, and a clear implication is that the substance must exist in the liquid state over a large range of temperatures. Second, it must be able to dissolve and transport other elements. After all, inability to effectively transport other atoms was the reason that solid and gaseous solvents were rejected.
On Earth, the universal solvent of life is water. This miraculous substance may not be a universal solvent, but it is useful to discuss water’s great properties so to understand what sorts of features other potential solvents must possess.
The covalent bonds we’ve already examined are not the only types of molecular bonds that are possible. Another important type of bond is called an ionic bond. While in a covalent bond, two adjacent atoms will share electrons; in an ionic bond, one atom will donate an electron to another atom. This causes one atom to have a positive charge and the other a negative one. The two atoms are then bound together by their respective charges. Common salt (sodium chloride) is like this.
Water molecules are an example of a polar molecule. This means that, even though they have no net electric charge, the electric charge inside them is not distributed equally. Thus one side of the molecule is, electrically speaking, “more negative,” while the other side is “more positive.” The interaction between the two sides of the water molecules and the molecules bound together with ionic bonds can break up the ionically bound molecules. In the case of salt, it isn’t salt molecules that are present in water when you dissolve salt, but rather freely floating sodium and chlorine atoms. We see this in
figure 6.11
. This wouldn’t be possible if water were not a polar molecule.
The electric charges from atoms set up electric fields, the means by which the atoms are attracted to one another. Water is able to shield electric fields very effectively, which is one of the reasons it can dissolve things so well. The dissolved atoms (say the positively charged sodium and negatively charged chlorine) are not able to see each other. Were they able to see one another, they would be attracted and recombine. This property of matter is called a “dielectric constant,” and it is very large for water with a numeric value of 80, which means that water can dissolve 80 times more of a solute than it would be able to otherwise. Water also can break up while in a liquid form, both donating and accepting a hydrogen atom, making OH
–
(hydroxide, a base) or H
3
O
+
(hydronium, an acid). The existence of acids and bases can be crucial for many chemical reactions relevant to life.
FIGURE 6.11
.
Water is a polar molecule, which means the arrangement of hydrogen and oxygen atoms causes one side of the molecule to have a slight positive charge, while the other has a negative charge. This property helps water dissolve materials held together by an ionic bond, like common salt, or sodium chloride (NaCl), shown here.
Water is liquid over a temperature range of 180°F, or 100°C. This range is quite large and will become important in a following chapter when we look at the concept of a planetary habitable zone. This is the range of distances from a star where the solvent (in our case water) will remain liquid.
Water has another hugely useful property. It takes a tremendous amount of heat to change its temperature. If you live anywhere near a coast, you know that the temperature at the beach is cooler in the summer and warmer in the winter than the surrounding areas. This is because on a terribly hot summer day, when the sun is beating down on you and you think you’re going to melt, the water tends to be cooler than the air. While the sun is shining on you, it is also shining on the water. However water needs to absorb a (relatively) huge amount of energy to change its temperature, so it stays cool (and thereby cools the area near the beach, kind of like sitting next to the refrigerator with the door open). To assign a number, it is five times easier to heat up sand than water.
Similarly, in the winter, as a wintery north wind blows through you, biting cold, a large reservoir of water will contain considerable heat. This is the reason that the North Atlantic remains free of ice so far north, while the air is teeth-chatteringly cold. In a reverse of the concerns of the summer, because of the properties of water, the ocean has to lose a lot more energy to change its temperature.
Water has even more helpful and unusual properties. In addition to liquid water’s being essentially a huge heat sponge, it takes a lot of energy to melt ice (and a corresponding large amount of energy must be given off to freeze water). Similarly, a large amount of energy is involved in converting water to steam, and vice versa. These properties are essential in the thermal regulation of the surface of the Earth.
Yet another curious feature of water is that, unlike most other substances, the solid phase of water (ice) has a lower density than the liquid phase. Basically, ice floats. Consider what would happen if the converse were true. When the weather got cold, ice would freeze and then sink to the bottom of the lake or ocean. As the ice descended, it would melt some but, in doing so, would cool the water below. Eventually the bottom water would be nearly the temperature of ice. Further melting and sinking would leave ice on the bottom of the body of water. After that, year after year, the ice would sink, building up the ice thickness until the lake or ocean was frozen solid, with only a small portion of the surface where there would be seasonal thawing of the water. The poles of the Earth would be frozen solid, from the ocean floor to near the surface.
However, real ice floats and insulates the water below from the colder air. Again, ice helps regulate the temperature of the surroundings. Without water, the Earth’s environment would be very different.
Chemists have considered other possible solvents that at least have potential as a water replacement. One important consideration is the atmospheric pressure on the surface of the planet. We are necessarily somewhat biased, inasmuch as the pressure on the surface of the Earth seems normal. In contrast, the surface pressure on Venus is 92 times the pressure on Earth. At such pressures, other substances can be liquid over larger temperature ranges. For instance, on Venus, water can be liquid from 32 to 350°F.
For the following discussion, we limit ourselves to one earth atmosphere of pressure. At our familiar pressure, the following substances have been considered as possible solvents: water, ammonia, hydrogen fluoride, and methane (
table 6.1
).
TABLE 6.1
.
Comparison of possible solvents