Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (568 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

Current serum (collected within 2 weeks).
Historic serum if the patient is sensitized with preference to serum that contains DSA that were defined by single antigen bead assay (collected within 6 months, preferably).
The serum sample collected 2–3 weeks after a known sensitizing event should be included in cross-match if available. Most often, the current sample would meet this requirement.
Results of the final cross-matches are reported to the transplant program before renal transplantation or combined organ and tissue transplants in which a kidney is to be transplanted, except for emergencies. If emergency transplants are performed before the cross-match test results are available, information provided by the transplant candidate’s physician to the laboratory as to the reason for the emergency transplant is documented.
DSA:
D
onor-
s
pecific
a
ntibody is determined based on the patient’s HLA antibody profile and the potential donor’s HLA type. The DSA specificity can change when patients change their potential donors. Since patients and donors are fully typed during initial evaluation, HLA antibody analysis can identify DSA for each patient–donor pair.
E. Deceased donor transplant HLA testing
Deceased kidneys must be allocated according to the UNOS policies. The final decision to accept a particular organ will remain the choice of the transplant surgeon and/or physician responsible for the care of the candidate. This allows physicians and surgeons to exercise their medical judgment regarding the suitability of the organ being offered for a specific candidate, to be faithful to their personal and programmatic philosophy about such controversial matters as the importance of cold ischemia time and anatomic anomalies, and to give their best assessment of the prospective recipient’s medical condition at the moment. If an organ is declined for a candidate, a notation of the reason for that decision must be made on the appropriate form and submitted to the UNOS promptly.
The minimum typing requirements for both recipient and donor are antigens. When reporting DR antigens, DRBI and DRB3/4/5 must be reported. The lab is encouraged to report splits for all loci.
Calculated PRA (cPRA) is the percentage of donors expected to have one or more of the unacceptable antigens indicated on the waiting list for the candidate. Sensitized waiting list candidates with defined unacceptable HLA antigens that yield a cPRA of 80% or greater will be assigned points on the waitlist based on the current allocation policy. Each transplant center may define the criteria for unacceptable antigens that are considered as contraindications for transplantation. Unacceptable antigens that are defined by laboratory detection of HLA-specific antibodies must be determined using at least one solid-phase immunoassay using purified HLA molecules. It is the prerogative of the transplant center to establish criteria for additional unacceptable antigens, such as repeat transplant mismatches. The cPRA will be calculated automatically when the unacceptable antigens are listed or updated on the waiting list. The cPRA will be derived from the HLA antigen/allele group and haplotype frequencies for the different racial/ethnic groups in proportion to their representation in the national deceased donor population.
A prospective cross-match is mandatory for all candidates, except where clinical circumstances support its omission. The transplant program and its histocompatibility laboratory must have a joint written policy that states when the prospective cross-match may be omitted. Guidelines for policy development, including assigning risk and timing of cross-match testing, are set out in UNOS Appendix D to Policy 3.
F. UNET waitlist maintenance and transplant support
The UNOS has developed an online database system, called UNET, to collect, store, analyze, and publish all Organ Procurement and Transplantation Network (OPTN) data that pertain to the patient waiting list, organ matching, and transplants. Launched on October 25, 1999, this system contains data regarding every organ donation and transplant event occurring in the United States since 1986. UNET is a fail-safe, 24/7, secure internet-based transplant information database. It enables the nation’s organ transplant institutions to register patients for transplants, match donated organs to waiting patients, and manage the time-sensitive, life-critical data of all patients before and after their transplants.
When a new patient is listed in the UNET by the Transplant Program, the data will be verified by HLA staff using most current typing and antibody results. Double review is required for any UNET edits, and the documentation will be signed and stored in the patient file indefinitely.
As required by the UNOS, the UNET Waitlist histocompatibility data for each patient for whom the laboratory performed testing are reviewed and verified monthly or as needed by the HLA lab staff. Documentation of such reviews is kept for at least 3 years or the interval required by local, state, and Federal regulations, whichever is longer. They are available for audit by the UNOS. The unacceptable antigen list is uploaded to the UNET each month to establish cPRA value.
G. Posttransplant monitoring
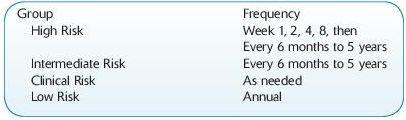
Risk Stratification:
High risk: DSA+, both to live or deceased current donor
Intermediate risk: DSA- and cPRA >80%, HLA-mismatched transplant only
Low risk: DSA- and cPRA <80% or 0 mismatched transplant
Clinical risk: prior transplant; low-risk patient with clinical indications
Monitoring schedule:
Acute Renal Allograft Rejection
