The Cerebellum: Brain for an Implicit Self (16 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

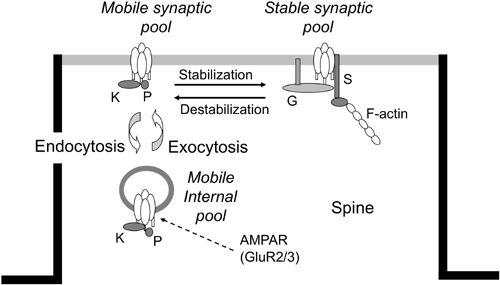
Figure 20. AMPAR trafficking in parallel fiber-Purkinje cell synapses.
A schematic of the three-compartmental kinetic model of the constitutive trafficking of AMPAR receptors (see AMPAR at arrow), which consist of GluR2/3 subunits. In the postsynaptic membrane (half dark line), the stable form of AMPARs (on the right) changes to the mobile form (on the left) and vice versa. In the resting state, they are in equilibrium. The mobile forms of AMPARs in the synaptic membrane are also in equilibrium with their mobile forms in the internal pool. Symbols attached to AMPAR models represent GRIP (G), PICK1 (K), phosphor (P), and stargazin (S). (Courtesy of Kazuhiko Yamaguchi.)
This section focuses on a number of other molecules related to LTD induction. It includes some molecules whose roles in LTD induction are now emerging but require more definitive study.
Nitric oxide (NO).
Parallel-fiber impulses activate NO synthase in parallel fibers, which releases NO that then diffuses to the postsynaptic side of parallel fiber-Purkinje cell synapses (
Shibuki and Kimura, 1997
). NO then activates a cascade that links guanylyl cyclase, cyclic GMP, and protein kinase G (PKG) and results in the phosphorylation of the G-substrate in Purkinje cells. G-substrate has been cloned, and its molecular structure has been determined (
Endo et al., 1999
;
Hall et al., 1999
). The phosphorylated G-substrate is a potent inhibitor of two protein phosphatases, PP1 and PP2A (
Detre et al., 1984
), and hence disinhibits kinases involved in conjunctive LTD induction (
Endo et al., 2003
).
Two sets of observations in slice preparations indicate the involvement of NO in conjunctive LTD induction. First, this induction occurred following the bath application of an NO donor, sodium nitroprusside (
Shibuki and Okada, 1991
), or infusion of the NO donor, 4-ethyl-2-hydroxyamino-5-nitro-hexenamide (NOR3) into Purkinje cells (
Daniel et al., 1993
). LTD also occurred after the release of NO from its caged form within Purkinje cells in combination with depolarization-induced Ca
2+
entry (
Lev-Ram et al., 1997a
). Second, the induction of conjunctive LTD was readily blocked by bath application of NO synthase inhibitors (
Crepel and Jaillard, 1990
;
Shibuki and Okada, 1991
;
Daniel et al., 1993
). LTD induced by conjunctive stimulation of parallel fibers and membrane depolarization was also blocked by KT5823, a PKG inhibitor (
Reynolds and Hartell, 2001
). The targeted disruption of the NO synthase gene in mice has been shown to result in the near-complete loss of NO synthase activity in the cerebellum (
Huang et al., 1993
). As a result, conjunctive LTD did not occur in cerebellar slices obtained from these mice (
Lev-Ram et al., 1997b
). Disappointingly, however, the photolytic uncaging of NO and cyclic GMP inside Purkinje cells did not rescue LTD in these slices. A possible explanation for this failure is that a prolonged absence of NO synthase altered the signaling pathway downstream of cyclic GMP.
Note that NO is required for LTD induction in cerebellar slices but not in cultured Purkinje cells. It has been shown that reagents that stimulate (sodium nitroprusside) or inhibit (hemoglobin, NG-nitro-l-arginine) NO signaling do not affect the reduced form of conjunctive LTD in cultured Purkinje cells (
Linden and Connor, 1991
). Also, LTD in cultured Purkinje cells derived from mice deficient in the neuronal isoform of NO synthase is indistinguishable from that in cultures from wild-type mice (
Linden et al., 1995
). This suggests that NO is not an indispensable mediator of conjunctive LTD, but is required to help mediators under certain conditions, such as in a slice preparation but not in a culture (
Ito, 2002
). Concerning the role of the NO-cyclic GMP-G-substrate cascade, a complication is that G-substrate-deficient mutant mice exhibit a unique age-dependent conjunctive LTD that is abolished at postnatal weeks 5 and 6. Before or after these weeks conjunctive LTD occurs normally (
Endo et al., 2009
). This finding indicates that the NO-cyclic GMP-G-substrate cascade is also a modulator (not mediator) of conjunctive LTD because it is required for only a few postnatal weeks.
δ2 receptors.
The glutamate receptor δ2 (GluRδ2) is an orphan receptor predominantly expressed in Purkinje cells, in contrast to the δ1-subtype, which is found widely throughout the adult brain, but at low levels. The immunogold-labeled δ1/2-subunits of glutamate receptors are present in parallel fiber-Purkinje cell synapses with a distribution pattern similar to AMPA receptors (Landsend et al., 1993;
Petralia et al., 1998
). In the dendritic spines of Purkinje cells, δ-receptors are anchored to actin filaments via spectrin, an actin-binding protein (
Hirai and Matsuda, 1999
;
Hirai, 2000
). However, when expressed alone or with other glutamate receptors, GluRδ2 does not form functional glutamate-gated ion channels, nor does it bind to glutamate analogs. Therefore, how GluRδ2 participates in cerebellar functions remains an open issue.
Very recently, the ligand of δ2 receptors has been identified as cerebellin 1, a peptide secreted from granule cells. It binds directly to the N-terminal domain of GluRδ2. Exogenous application of cerebellin 1 to postsynaptic cells expressing GluRδ2 induces new synapses
in vitro
and in the adult cerebellum
in vivo
(
Matsuda et al., 2010
). Furthermore, another study revealed that GluRδ2 mediates cerebellar synapse formation by interacting with presynaptic neurexins through cerebellin 1 (
Uemura et al., 2010
).
A role for δ2-receptors in LTD induction is suggested by three major findings. (1) Treatment of cultured Purkinje cells with an antisense oligonucleotide against the δ2-subunit mRNA blocked LTD induction without other noticeable effects on Purkinje cells (
Hirano et al., 1994
;
Jeromin et al., 1996
). (2) Purkinje cells in cerebellar slices (Kashiwabuchi et al., 1995) and tissue cultures (
Hirano et al., 1995
) derived from δ2-deficient gene-knockout mice did not exhibit LTD. (3) An antibody specific for the putative ligand-binding region of GluRδ2 induced AMPA receptor endocytosis, attenuated synaptic transmission, and abolished LTD (
Hirai et al., 2003
). Another open question is how the role of GluRδ2 in LTD is related to that in synapse formation and maintenance.
Protein tyrosine kinases.
The nonreceptor-coupled type of protein tyrosine kinase activity, a component of the intracellular signaling cascade in general, is prominent in the cerebellum. Purkinje cells express neuronal isoforms of c-src protein tyrosine kinase, pp60c-src (+) (
Sugrue et al., 1990
) and pp62c-yes, both being members of the src family (
Zhao et al., 1991
). Purkinje cells are also rich in tyrosine phosphatase, which antagonizes protein tyrosine kinases (
Levy et al., 1993
). Two protein tyrosine kinase inhibitors (lavendustin and herbimycin A) block conjunctive LTD (
Boxall et al., 1996
). Herbimycin A also prevents the depression of parallel fiber-EPSPs produced by intracellular infusion of a PKC activator, (-)-indolactan V. These findings indicate that protein tyrosine kinases, operating in association with PKC, are required for LTD induction. Protein tyrosine kinases may interact with PKC directly, but an indirect interaction via the G protein-PLC-DAG pathway is also possible because protein tyrosine kinase inhibitors block the Gq/11 protein-coupled receptor-mediated formation of IP
3
(
Umemura et al., 1999
).
Ca
2+
/calmodulin-dependent protein kinase (CaMKII).
This serine/threonine protein kinase is expressed at high concentrations and preferentially in neurons. One of the two isoforms, α, is predominantly expressed in the forebrain (a:b ratio, 3:1), whereas the other, β, is expressed in the cerebellum (α:β ratio, 1:4) (
Miller and Kennedy, 1985
). In the cerebellum, CaMKIIα is specifically concentrated in Purkinje cell somata and dendrites (
Walaas et al., 1988
). CaMKIIβ appears to function as an F-actin targeting molecule for localizing CaMKIIα/β hetero-oligomers to dendritic spines (
Shen et al., 1998
). Even though arachidonic acid and its metabolites are known to inhibit CaMKII (
Piomelli et al., 1989
), the involvement of CaMKII in conjunctive LTD has been unclear. A very recent report has suggested, however, that CaMKIIβ is important in controlling the direction of plasticity at parallel fiber-Purkinje cell synapses because a stimulus that induces synaptic depression in wild-type mice results in synaptic potentiation in CaMKIIβ knockout mice and vice versa. Possibly, this enzyme regulates calcium signals to produce synaptic depression or potentiation (
van Woerden et al., 2009
).
Protein phosphatases.
Two major types of serine/threonine-specific protein phosphatase are PP1 and PP2. PP1 specifically dephosphorylates the b-subunit of phosphorylase kinase and is inhibited by inhibitor-1 and inhibitor-2. In contrast, PP2 dephosphorylates the a-subunit of the phosphorylase kinase preferentially and is unaffected by the above-mentioned inhibitors. PP2 comprises three enzymes; PP2A, PP2B, and PP2C (
Cohen, 1989
).
PP2A, like PP1, does not require cations for its activity and is sensitive to okadaic acid, whereas PP2B, also called calcineurin, is Ca
2+
/calmodulin-dependent and much less sensitive to okadaic acid. PP2C is Mg
2+
-dependent and insensitive to okadaic acid. PP1 consists of isoforms with different catalytic units. A strong immunoreactivity for a catalytic unit of PP1, PP1γ1, but not PP1δ or PP1α, has been observed in Purkinje cells, including their fine dendritic branches and spines (
Hashikawa et al., 1995
). Immunoreactivity for PP2A (α and/or β) was observed in Purkinje cell somata and thick dendrites, but not in the fine dendrites and spines. PP2Aα and PP2Aβ mRNAs are expressed in Purkinje cells (
Abe et al., 1994
). PP2B is present in the cerebellum (
Sola et al., 1999
). The phosphorylated G-substrate is a potent inhibitor of PP1 and PP2A (
Endo et al., 2003
). As would be expected, specific inhibition of postsynaptic PP2A by fostriecin or cytostatin induced a gradual and use-dependent decrease of synaptic current evoked by the stimulation of a single granule cell mimicking conjunctive LTD (
Launey et al., 2004
).
c-Fos and jun-B.
The induction of a reduced form of conjunctive LTD by the coadministration of AMPA and 8-Br-cyclic GMP to cerebellar slices is accompanied by an enhanced expression of c-Fos and Jun-B in Purkinje cells (
Nakazawa et al., 1993
). This suggests a role for active transcriptional complexes such as c-Fos/Jun-B in LTD induction. Application of the same compounds to the surface of the cerebellum
in vivo
, in conjunction with electrical stimulation of climbing fibers, induced the expression of Jun-B in Purkinje cells (
Yamamori et al., 1995
). Climbing fiber-parallel fiber conjunctive stimulation in the cerebellum
in vivo
also induced Jun-B expression, which was blocked by a nitric oxide synthase inhibitor (
Yano et al., 1996
). Jun-B/c-Fos forms an AP-1 complex, which acts as a transcriptional factor (
Morgan and Curran, 1989
). Possibly, this transcriptional factor plays a role in conjunctive LTD induction.
Glial fibrillary acidic protein (GFAP).
This intermediate filament protein is specifically expressed in astrocytes. In the cerebellum, it is expressed substantially in Bergmann glia, a subset of astrocytes. GFAP-deficient mice exhibit normal cerebellar architecture and synaptic transmissions, but not conjunctive LTD (
Shibuki et al., 1996
). What is missing in GFAP-deficient mice in terms of signal transduction for LTD induction is as yet unclear. However, because the membranes of Bergmann glia possess AMPA receptors and glutamate transporters, it may well be assumed that those of the Bergmann glia that respond to parallel fiber and/or climbing fiber activation secrete an as-yet-unknown diffusible factor, which may be required for LTD induction.
Corticotropin-releasing factor (CRF).
This neuropeptide containing 41 aminoacids is generally known as a stress hormone that plays a role in responses of the body to stress and is involved in affective disorders. CRF is contained in climbing fibers and cells of their origin in the inferior olive (IO), whereas Purkinje cells highly express CRF receptor type 1 (CRFR1) mRNA (Potter et al., 1994). Because specific CRFR1 antagonists, α-helical CRF-(9-41) and astressin, effectively blocked conjunctive LTD induction, CRF released from climbing fibers is critically involved in LTD-inducing mechanisms at parallel fiber synapses (
Miyata et al., 1999
). As would be expected, LTD was no longer observed when climbing fibers had been deprived by 3-aminopyridine intoxication, and it was restored by CRF replenishment. Because CRFR1 antagonists blocked LTD induced by conjunction of parallel fiber stimulation and depolarizing pulses without stimulating climbing fibers, it appears that CRF spontaneously released from climbing fibers, regardless of conjunctive stimulation, is sufficient for LTD induction. CRF release from climbing fibers may be a kind of stress response because errors represented by
climbing fiber impulses imply discrepancy between intended and actual movements, and between demand for precision and discontent about inaccurate performance (
Ito, 2009
). However, what mechanisms organize these activities is unclear when climbing fibers and other CRF-containing neurons located in the amygdala and hypothalamus have no obvious neuronal connections.
