The Case for Mars (32 page)

Plastics are, of course, among the most central materials of modern life. They can be made on Mars because of the ubiquitous presence there of carbon and hydrogen. This should give pause to those who believe the prospects for settlement on the Moon are superior to those on Mars. The Moon has no significant quantities of carbon and hydrogen; they exist there only in parts per million quantities, somewhat like gold in seawater. The manufacture of cheap plastics will never be possible on the Moon. In fact, on the Moon for a long time to come, plastics would literally be worth their weight in gold.
MAKING CERAMICS AND GLASS
Clay-type minerals are also ubiquitous in the Martian surface soils, making the manufacture of ceramics for pottery and similar purposes a straightforward enterprise. The most common material measured by the
Viking
landers on Mars, though, was silicon dioxide, SiO
2
. Making up about 40 percent of both the
Viking 1
and 2 soil samples by weight, silicon dioxide is the basic constituent of glass, which thus can readily be produced on Mars using sand-melting techniques similar to those that have been used on Earth for thousands of years. Unfortunately for the Martian glass industry, however, the second most common compound (about 17 percent in the
Viking
samples) is iron oxide, Fe
2
O
3
. This poses a problem. If you want to make optical-quality glass, the sand used as feedstock must be nearly iron free, and such sand may well be hard to come by on Mars. So, if you want to make optical glass on Mars, you first need to remove the iron oxide. This can be done by hitting the iron oxide with hot carbon monoxide “waste” from your RWGS reactor, thereby reducing it to metallic iron and carbon dioxide, and then removing the iron metal product with a magnet. Tiresome, I admit, but you get to keep the iron for other uses
, such as making steel, as will be discussed shortly. In fact, since the base will almost certainly need much more steel than optical glass, after the base foundry has been functioning for a while there should be no shortage of iron-denuded material for the glass makers to work with. It should be noted, however, that optical-quality glass is not required to make many important glass products, including fiberglass, an excellent material for constructing various types of structures.
TAPPING WATER
In the Martian mind, there would be one question perpetually paramount to all the local labor, women’s suffrage, and Eastern questions put together—the water question. How to procure water enough to support life would be the greatest communal problem of the day.
—Percival Lowell,
Mars
, 1895
Percival Lowell may have been wrong about a lot of things, but he was certainly prescient in his comment regarding water on Mars. From making rocket fuel, rover fuel, and oxygen, to producing plastics, bricks, mortar, and pottery; to growing crops, to sealing leaks and hardening soil with artificial permafrost, all the opportunities to open Mars to human exploration and settlement we have discussed so far depend upon water. While the logistics of transporting water to Mars is hopelessly unattractive, for our first several missions we can afford to
make
water, bringing only its 11 percent hydrogen component from Earth to combine with the oxygen in Mars’ carbon dioxide atmosphere. But once the base-building phase begins, we must move beyond this. The increased propellant requirements deriving from the expanded level of human activity, the multitude of additional civil and chemical engineering uses, and above all the agricultural requirements that arise during the base-building phase, all conspire to increase water demand on Mars far beyond the point where hydrogen transport from Earth remains a viable option. If human civilization is ever to grow on Mars, we must find a way to obtain indigenous water.
So, if we are wise, we will position our base near where water is likely to be found. On Mars, this probably means the northern hemisphere. If you look at Mars today, you’ll see a large region of de
pressed topography in the Martian Arctic that contains very few craters. It is believed that in Mars’ early history, this vast basin was filled with water, which shielded the surface from meteor impact during the planet’s first billion or so years. The last remnant of this ancient ocean is the northern polar cap, which is made of water ice (about two million cubic kilometers of it, by current estimates
24
). In addition, we see from orbital images that the north boasts many more dry river beds and out
flow channels than the south. It is likely that when these channels flowed their last, deposits of ice or permafrost were left at their mouths. These deposits may still exist, hidden from our view by a layer of dust. Measurements of atmospheric humidity taken from orbit also leave no doubt that the northern hemisphere is wetter than the southern, with the wettest time of the year being the northern spring. The existence of much larger amounts of water in the northern hemisphere’s past is also significant to future Martian colonists for another reason; hydrological activity is key to the formation of a large variety of mineral ores. If Horace Greeley had lived on Mars, his advice to young Martians seeking their fortune would have been simple—Go north.
There are a number of possible ways to get water on Mars. The first, most attractive, but most problematical method is simply to find it. As discussed in Chapter 6, there may be subsurface, geothermally heated pools of liquid water on Mars. Such pools could be detected within a kilometer of the surface by rover crews equipped with ground penetrating radar. The rover crews won’t have to search randomly. Low-resolution radar investigations conducted from orbit or from balloon-borne probes can identify in advance the best places to look. If we find such a pool and drill down to it, the hot pressurized water should come shooting out of the ground like a Texas oilfield gusher. Once it hits the low-pressure, cold Martian air, the water won’t stay warm for long. Depending upon its speed of ejection, it will probably freeze into ice crystals and fall back to the ground before it has gone a hundred meters. In no time at all a snow volcano could form, possibly of considerable size. Extracting the water in such a spectacular way would be rather wasteful though, because such a hydrothermal well could also represent a significant source of power. But as far as water
access is concerned, next to siting the base over a hot artesian well, this is about as good as it gets.
Of course, things might not work out so well. Subsurface liquid water within drilling range may not be found. What then? Well, the next best thing would be to find brines. Saturated salt solutions can be liquid at temperatures as low as -55°C, which means that even without geothermal heat, such liquid brines, protected from evaporation by a modest layer of soil or ice, could exist on Mars today very close to the surface. In addition to being a good source of water, brines would be of great interest as candidate sites for finding extant Martian life. No brines have been identified on Mars as yet, but salts certainly have, and some scientists believe that light-colored features surrounding certain basins imaged on Mars may represent large salt deposits left behind on the shore lines of vanished Martian seas.
After brines, the next most interesting source of water on Mars would be ice. There are large deposits of water ice on Mars’ north polar cap, but that’s not where we are going to build our base. We see no large permanent deposits of ice south of 75° north latitude, but theory indicates that poleward of 40° N, underground ice should be stable within a meter of the surface. There may also be local anomalies. Where I live in Colorado, it can be winter on the north side of the house while it is summer on the south side, and it is not uncommon even on a blistering, mid-August day to come across snow nestled in a shady depression of a hill’s northern side. Without a doubt in some cold crevice, lava tube, or cavern on the north face of some hill on Mars there is ice to be found, and in regions where planetary-scale climate models say it can’t be. If you want to harvest some, though, bring dynamite. Ice at Martian temperatures can be pretty tough stuff. Still, a pure ice deposit in a nonpolar region would be a rare find. It’s much more likely that Martian explorers would come across permafrost, or frozen mud. Permafrost can be very strong. In fact for some applications it’s the ideal material for construction on Mars. A permafrost brick would be much stronger than a hot fired red clay brick, and you don#8217;t need an oven to make one or use mortar to get one brick to adhere to the next. Instant rock, just add water. Bring lots of dynamite.
So much for the heroic forms of Martian water-prospecting and mining. Let’s take a look now at some more mundane, industrial-style methods.
Martian soil has some water in it. We know that for a fact because at both
Viking
landing sites, random samples of soil scooped from the top 10 centimeters of the surface emitted about 1 percent of their weight in water when heated to 500°C. That’s not too bad, but in fact the test was unfairly skewed, because surface soil is the driest there is; the samples were heated for only 30 seconds; and furthermore, the samples were held in an unsealed vessel at 15°C for days before the test. Since 15°C is much warmer than average for Mars, the odds are very high that a significant amount of water was lost from the samples via outgassing prior to the test. On the basis of the
Viking
results, it would be a good bet that
average
Martian soil is at least 3 percent water. But some soils are likely to be much wetter than this average. For example, there are salts on Mars that typically contain up to 10 percent chemically bound water that can be released by heating to appropriate temperatures. Clays, which are common on Mars, also have excellent water adsorption capacities. For example, smectite clays have been found in SNC meteorites. Smectite clay is known commonly as “swelling clay,” because it can absorb several tens of percent of its weight in water and will swell in the process. The mineral gypsum (CaSO
4
· 2H
2
O) has been also been found in many SNC meteorites. It’s likely that gypsum is quite common on Mars, because sulfur and calcium concentrations measured at both
Viking
landing sites were much higher (forty and three times, respectively) than their averages in soils on Earth. Gypsum can be over 20 percent water by weight.
Whether 3 percent or 20 percent, to get this water out of the soil all that is needed is heat. This can be done in one of two ways—either you bring the soil to the heater or the heater to the soil. The first of these methods is illustrated in
Figure 7.3
. A truck loaded with some relatively wet soil dumps its load onto a conveyor belt leading into an oven. The oven heats the soil to 500°C or so, causing the adsorbed water to outgas. The steam so produced is collected in a condenser, while the dehydrated dirt is dumped. The resulting “slag heap”
is an inconvenience, but the energetics of this system aren’t too bad. If soil with a 3 percent water content is used as a feedstock, the energy required to run the system is about 3.5 kWh (kilowatt hours) of heat for every kilogram of water produced.
25
At that rate a 100 kWe (kilowatt-electric) reactor could produce 700 kg/day of water if its elect
ricity is used to power the oven, or up to 14,000 kg/day of water if the reactor’s waste heat is used to bake the dirt. (Thermoelectric generators used on today’s space nuclear power sources are only 5 percent efficient at turning their power into electricity; the other 95 percent comes out as “waste heat.”)
Alas, but there is that annoying waste pile of dried dirt. We could make 14,000 kg/day of water, but we’d be piling up 462,000 kg/day of desiccated “slag.” That might be acceptable—it’s only about 120 cubic meters, or six truckloads, of material. May Be we can use the slag for something; may be we can just dump it in a nearby crater.
FIGURE 7.3
Truck, oven, and slag pile system or extracting water from Martian soil. (Artwork by Michael Carroll.)
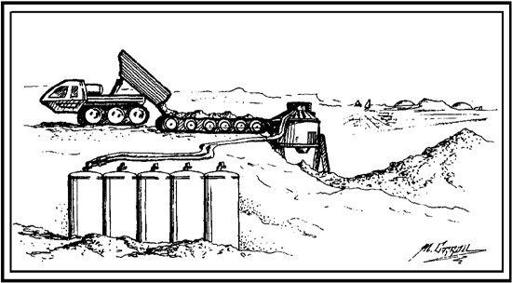
But, if you don’t want to move all that dirt around, the alternative is to bring the heater out to the field. One way that’s been suggested to do this is to have a mobile oven that wheels along, ingesting soil, baking it, condensing the steam, and ejecting the desiccated dirt as it travels.
20
You probably would
n’t want to use a nuclear reactor for such a system, but, instead, a radioisotope thermoelectric generator (RTG) such as has been used on
Voyager, Viking, Galileo
, and other outer solar system spacecraft. The standard RTG puts out 300 watts of electricity, enough to move the cart, along with 6 kW of waste heat, sufficient to produce 42 kilograms of water a day from 3 percent grade feedstock. Such a unit would be quite handy to small crews operating out in the field, or as an adjunct piece of equipment for early exploration missions (42 kilograms of water produced daily over the course of a single, 500-day Mars Direct mission surface stay adds up to 21,000 kilos of water), but its output is quite small relative to the needs of a large developing Mars base. Of course, we could produce all the water we need by operating a multitude of them, but all those RTGs would be expensive, and anyway we’d still be moving a lot of dirt, pebbles, and rocks around, with all the wear and tear on the involved equipment that implies. Is there a gentler approach?
