Ross & Wilson Anatomy and Physiology in Health and Illness (29 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

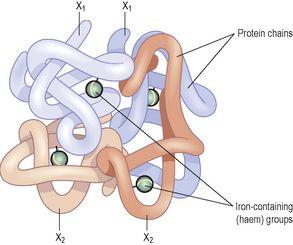
Figure 4.5
The haemoglobin molecule.
Iron is carried in the bloodstream bound to its transport protein,
transferrin
, and stored in the liver. Normal red cell production requires a steady supply of iron.Iron absorption from the alimentary canal is very slow, even if the diet is rich in iron, meaning that iron deficiency can occur readily if losses exceed intake.
Oxygen transport
When all four oxygen-binding sites on a haemoglobin molecule are full, it is described as
saturated
. Haemoglobin binds reversibly to oxygen to form oxyhaemoglobin, according to the equation:
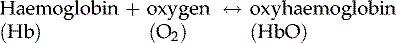
As the oxygen content of blood increases, its colour changes too. Blood rich in oxygen is bright red because of the high levels of oxyhaemoglobin it contains, compared with blood with lower oxygen levels, which is dark bluish in colour because it is not saturated.
The association of oxygen with haemoglobin is a loose one, so that oxyhaemoglobin releases its oxygen readily, especially under certain conditions.
Low pH
Metabolically active tissues, e.g. exercising muscle, release acid waste products, and so the local pH falls. Under these conditions, oxyhaemoglobin readily breaks down, giving up additional oxygen for tissue use.
Low oxygen levels (hypoxia)
Where oxygen levels are low, oxyhaemoglobin breaks down, releasing oxygen. In the tissues, which constantly consume oxygen, oxygen levels are always low. This encourages oxyhaemoglobin to release its oxygen to the cells. In addition, the lower the tissue oxygen level, the more oxygen is released, meaning that as tissue oxygen demand rises, so does the supply to match it. On the other hand, when oxygen levels are high, as they are in the lungs, oxyhaemoglobin formation is favoured.
Temperature
Actively metabolising tissues, which have higher than normal oxygen needs, are warmer than less active ones, which drives the equation above to the left, increasing oxygen dissociation. This ensures that very active tissues receive a higher oxygen supply than less active ones. In the lungs, where the alveoli are exposed to inspired air, the temperature is lower, favouring oxyhaemoglobin formation.
Control of erythropoiesis
Red cell numbers remain fairly constant, because the bone marrow produces erythrocytes at the rate at which they are destroyed. This is due to a homeostatic negative feedback mechanism. The hormone that regulates red blood cell production is
erythropoietin
, produced mainly by the kidney.
The primary stimulus to increased erythropoiesis is
hypoxia
, i.e. deficient oxygen supply to body cells. This occurs when:
•
the oxygen-carrying power of blood is reduced by, e.g., haemorrhage or excessive erythrocyte breakdown (haemolysis) due to disease
•
the oxygen tension in the air is reduced, as at high altitudes.
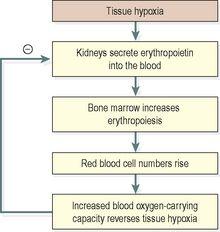
Hypoxia increases erythrocyte formation by stimulating erythropoietin production. Erythropoietin stimulates an increase in the production of proerythroblasts and the release of increased numbers of reticulocytes into the blood. It also speeds up reticulocyte maturation. These changes increase the oxygen-carrying capacity of the blood and reverse tissue hypoxia, the original stimulus. When the tissue hypoxia is overcome, erythropoietin production declines (
Fig. 4.6
). When erythropoietin levels are low, red cell formation does not take place even in the presence of hypoxia, and anaemia (the inability of the blood to carry adequate oxygen for body needs) develops.
Figure 4.6
Control of erythropoiesis: the role of erythropoietin.
Erythropoietin also regulates normal red cell replacement, i.e. in the absence of hypoxia.
Destruction of erythrocytes
The life span of erythrocytes is about 120 days and their breakdown, or
haemolysis
, is carried out by
phagocytic reticuloendothelial cells
. These cells are found in many tissues but the main sites of haemolysis are the spleen, bone marrow and liver. As erythrocytes age, their cell membranes become more fragile and so more susceptible to haemolysis. Iron released by haemolysis is retained in the body and reused in the bone marrow to form new haemoglobin molecules (
Fig. 4.4
).
Biliverdin
is formed from the haem part of the haemoglobin. It is almost completely reduced to the yellow pigment
bilirubin
, before being bound to plasma globulin and transported to the liver (see
Fig. 12.37, p. 303
). In the liver it is changed from a fat-soluble to a water-soluble form to be excreted as a constituent of bile.
Blood groups
Individuals have different types of antigen on the surfaces of their red blood cells. These antigens, which are inherited, determine the individual’s
blood group
. In addition, individuals make antibodies to these antigens, but not to their own type of antigen, since if they did the antigens and antibodies would react, causing a
transfusion reaction
, which can be fatal. These antibodies circulate in the bloodstream and the ability to make them, like the antigens, is genetically determined and not associated with acquired immunity.
If individuals are transfused with blood of the same group, i.e. possessing the same antigens on the surface of the cells, their immune system will not recognise them as foreign and will not reject them. However, if they are given blood from an individual of a different blood type, i.e. with a different type of antigen on the red cells, their immune system will mount an attack upon them and destroy the transfused cells. This is the basis of the transfusion reaction; the two blood types, the donor and the recipient, are
incompatible
.
There are many different collections of red cell surface antigens, but the most important are the ABO and the Rhesus systems.
The ABO system
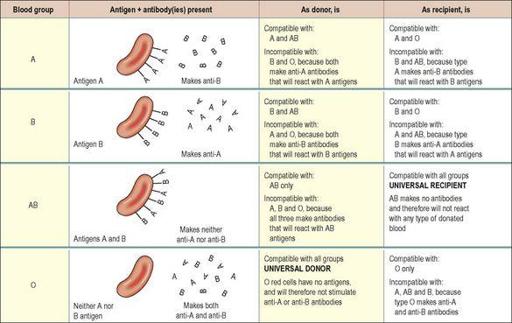
About 55% of the population has either A-type antigens (blood group A), B-type antigens (blood group B) or both (blood group AB) on their red cell surface. The remaining 45% have neither A nor B type antigens (blood group O). The corresponding antibodies are called anti-A and anti-B. Blood group A individuals cannot make anti-A (and therefore do not have these antibodies in their plasma), since otherwise a reaction to their own cells would occur; they do, however, make anti-B. Blood group B individuals, for the same reasons, make only anti-A. Blood group AB make neither, and blood group O make both anti-A and anti-B (
Fig. 4.7
).
Figure 4.7
The ABO system of blood grouping:
antigens, antibodies and compatibility.
Because blood group AB people make neither anti-A nor anti-B antibodies, they are sometimes known as
universal recipients
: transfusion of either type A or type B blood into these individuals is likely to be safe, since there are no antibodies to react with them. Conversely, group O people have neither A nor B antigens on their red cell membranes, and their blood may be safely transfused into A, B, AB or O types; group O is sometimes known as the
universal donor
. The terms
universal donor
and
universal recipient
are misleading, however, since they imply that the ABO system is the only one that needs to be considered. In practice, although the ABO systems may be compatible, other antigen systems on donor/recipient cells may be incompatible, and cause a transfusion reaction (
p. 69
). For this reason, prior to transfusion, cross-matching is still required to ensure that there is no reaction between donor and recipient bloods. Inheritance of ABO blood groups is described in
Chapter 17 (p. 433)
.
The Rhesus system
The red blood cell membrane antigen important here is the Rhesus (Rh) antigen, or Rhesus factor. About 85% of people have this antigen; they are Rhesus positive (Rh
+
) and do not therefore make anti-Rhesus antibodies. The remaining 15% have no Rhesus antigen (they are Rhesus negative, or Rh
−
). Rh
−
individuals are capable of making anti-Rhesus antibodies, but are stimulated to do so only in certain circumstances, e.g. in pregnancy (
p. 68
), or as the result of an incompatible blood transfusion.