Ross & Wilson Anatomy and Physiology in Health and Illness (11 page)
Read Ross & Wilson Anatomy and Physiology in Health and Illness Online
Authors: Anne Waugh,Allison Grant
Tags: #Medical, #Nursing, #General, #Anatomy

Figure 2.3
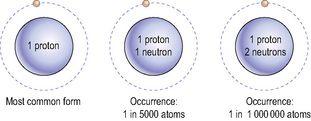
The isotopes of hydrogen.
Because the atomic weight of an element is actually an average atomic weight calculated using all its atoms, the true atomic weight of hydrogen is 1.008, although for most practical purposes it can be taken as 1.
Chlorine has an atomic weight of 35.5, because it contains two isotopes, one with an atomic weight of 35 (with 18 neutrons in the nucleus) and the other 37 (with 20 neutrons in the nucleus). Because the proportion of these two forms is not equal, the average atomic weight is 35.5.
Molecules and compounds
It was mentioned earlier that the atoms of each element have a specific number of electrons around the nucleus. When the number of electrons in the outer shell of an element is either the maximum number (
Fig. 2.1
), or a stable proportion of this fraction, the element is described as
inert
or chemically unreactive, i.e. it will not easily combine with other elements to form compounds. These elements are the inert gases – helium, neon, argon, krypton, xenon and radon.
Molecules
consist of two or more atoms that are chemically combined. The atoms may be of the same element, e.g. a molecule of atmospheric oxygen (O
2
) contains two oxygen atoms. Most molecules, however, contain two or more different elements, e.g. a water molecule (H
2
O) consists of two hydrogen atoms and an oxygen atom. As mentioned earlier, when two or more elements combine, the resulting molecule is referred to as a compound.
Compounds that contain the elements carbon and hydrogen are classified as
organic
, and all others as
inorganic
. Living tissues are based on organic compounds, but the body requires inorganic compounds too.
Covalent and ionic bonds
The vast array of chemical processes on which life is based is completely dependent upon the way atoms come together, bind and break apart. For example, the simple water molecule is a crucial foundation of all life on Earth. If water was a less stable compound, and the atoms came apart easily, human biology could never have evolved. On the other hand, the body is dependent upon the breaking down of various molecules (e.g. sugars, fats) to release energy for cellular activities. When atoms are joined together, they form a chemical bond that is generally one of two types:
covalent
or
ionic
.
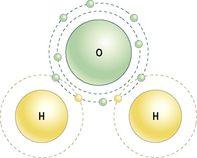
Covalent bonds are formed when atoms share their electrons with each other. Most molecules are held together with this type of bond; it forms a strong and stable link between its constituent atoms. A water molecule is built using covalent bonds. Hydrogen has one electron in its outer shell, but the optimum number for this shell is two. Oxygen has six electrons in its outer shell, but the optimum number for this shell is eight. Therefore, if one oxygen atom and two hydrogen atoms combine, each hydrogen atom will share its electron with the oxygen atom, giving the oxygen atom a total of eight outer electrons, making it stable. The oxygen atom shares one of its electrons with each of the two hydrogen atoms, so that each hydrogen atom has two electrons in its outer shell, and they too are stable (
Fig. 2.4
).
Figure 2.4
A water molecule, showing the covalent bonds between hydrogen (yellow) and oxygen (green).
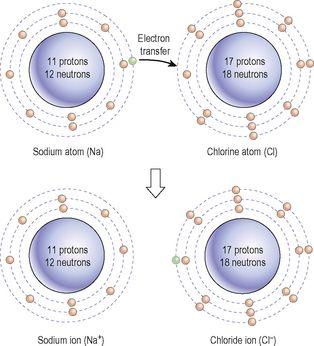
Ionic bonds are weaker than covalent bonds and are formed when electrons are transferred from one atom to another. For example, when sodium (Na) combines with chlorine (Cl) to form sodium chloride (NaCl) there is a transfer of the only electron in the outer shell of the sodium atom to the outer shell of the chlorine atom (
Fig. 2.5
).
Figure 2.5
Formation of the ionic compound, sodium chloride.
This leaves the sodium atom of the compound with eight electrons in its outer (second) shell, and therefore stable. The chlorine atom also has eight electrons in its outer shell, which, although not filling the shell, is a stable number. The sodium atom is now positively charged because it has given away a negatively charged electron, and the chloride ion is now negatively charged because it has accepted sodium’s extra electron. The two atoms, therefore, stick together because they are carrying opposite, mutually attractive, charges.
When sodium chloride is dissolved in water the ionic bond breaks and the two atoms separate. The atoms are charged, because they have traded electrons, so are no longer called atoms, but
ions
. Sodium, with the positive charge, is a
cation
, written Na
+
, and chloride, being negatively charged, is an
anion
, written Cl
−
. By convention the number of electrical charges carried by an ion is indicated by the superscript plus or minus signs.
Electrolytes
An ionic compound, e.g. sodium chloride, dissolved in water is called an
electrolyte
because it conducts electricity. Electrolytes are important body constituents because they:
•
conduct electricity, essential for muscle and nerve function
•
exert osmotic pressure, keeping body fluids in their own compartments
•
act as buffers to resist pH changes in body fluids.
Many biological compounds, e.g. carbohydrates, are not ionic, and therefore have no electrical properties when dissolved in water. Important electrolytes other than sodium and chloride include potassium (K
+
), calcium (Ca
2+
), bicarbonate (HCO
3
−
) and phosphate (PO
4
2−
).
Molecular weight
The molecular weight of a molecule is the sum of the atomic weights of the elements forming its molecules, e.g.:
Water (H
2
O)
| 2 hydrogen atoms | (atomic weight 1) | 2 |
| 1 oxygen atom | (atomic weight 16) | 16 |
| | Molecular weight | = 18 |
Sodium bicarbonate (NaHCO
3
)
| 1 sodium atom | (atomic weight 23) | 23 |
| 1 hydrogen atom | (atomic weight 1) | 1 |
| 1 carbon atom | (atomic weight 12) | 12 |
| 3 oxygen atoms | (atomic weight 16) | 48 |
| | Molecular weight | = 84 |
Molecular weight, like atomic weight, is expressed simply as a figure until a scale of measurement of weight is applied.
Molarity
This is the commonest way to express the concentration of many substances in body fluids.
The
mole
(mol) is the molecular weight in grams of a substance. One mole of any substance contains 6.023 × 10
23
molecules or atoms. For example, 1 mole of sodium bicarbonate (the example above) is 84 g.
In a
molar solution
, 1 mole of a substance is dissolved in 1 litre of solvent (dissolving fluid). In the human body the solvent is usually water. A molar solution of sodium bicarbonate is therefore prepared by dissolving 84 g of sodium bicarbonate in 1 litre of solvent.
Molar concentration may be used to measure quantities of electrolytes, non-electrolytes, ions and atoms provided the molecular weight of the substance is known. It means that a molar solution of a substance contains exactly the same number of particles as any other molar solution. If the molecular weight of the substance is not known, or if there is more than one material in solution, another system of measuring concentration has to be used, such as grams per litre. The tiny quantities of many substances dissolved in body fluids mean that physiological concentrations are often expressed as fractions of a mole: millimoles/litre (thousandths of a mole) or micromoles/litre (millionths of a mole) (
Table 2.2
).
Table 2.3
gives examples of the normal plasma levels of some important substances, given in molar concentrations and alternative units.
Table 2.2
Molar concentrations
| Solute units | Quantity per litre of solvent |
|---|---|
| 1 mole of sodium chloride molecules (NaCl) | 58.5 g |
| 1 millimole of sodium chloride molecules | 0.0585 g (58.5 mg) |
| 1 mole of sodium ions | 23 g |
| 1 micromole of sodium ions | 0.000023 g (23 μg) |
| 1 mole of carbon atoms | 12 g |
| 1 mole of oxygen gas (O 2 ) | 32 g |