Make: Electronics (5 page)
Authors: Charles Platt

Figure 1-26.
Think of the cells in a battery as being like two cylinders: one full of water, the other empty. Open a connection between the cylinders, and the water will flow until the levels are equal on both sides. The less resistance in the connection, the faster the flow will be.
Background
The man who discovered resistance
Georg Simon Ohm, pictured in Figure 1-27, was born in Bavaria in 1787 and worked in obscurity for much of his life, studying the nature of electricity using metal wire that he had to make for himself (you couldn’t truck on down to Home Depot for a spool of hookup wire back in the early 1800s).
Despite his limited resources and inadequate mathematical abilities, Ohm was able to demonstrate in 1827 that the electrical resistance of a conductor such as copper varied in inverse proportion with its area of cross-section, and the current flowing through it is proportional to the voltage applied to it, as long as temperature is held constant. Fourteen years later, the Royal Society in London finally recognized the significance of his contribution and awarded him the Copley Medal. Today, his discovery is known as Ohm’s Law.

Figure 1-27.
Georg Simon Ohm, after being honored for his pioneering work, most of which he pursued in relative obscurity.
Further Investigation
Attach the snap-on terminal cap (shown earlier in Figure 1-8) to the 9-volt battery. Take the two wires that are attached to the cap and hold them so that the bare ends are just a few millimeters apart. Touch them to your tongue. Now separate the ends of the wires by a couple of inches, and touch them to your tongue again. (See Figure 1-28.) Notice any difference?
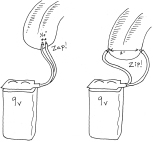
Figure 1-28.
Modifying the tongue test to show that a shorter distance, with lower resistance, allows greater flow of electricity, and a bigger zap.
Use your meter to measure the electrical resistance of your tongue, this time varying the distance between the two probes. When electricity travels through a shorter distance, it encounters less total resistance. As a result, the current (the flow of electricity per second) increases. You can try a similar experiment on your arm, as shown in Figure 1-29.
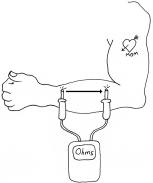
Figure 1-29.
Moisten your skin before trying to measure its resistance. You should find that the resistance goes up as you move the meter probes farther apart. The resistance is proportional to the distance.
Use your meter to test the electrical resistance of water. Dissolve some salt in the water, and test it again. Now try measuring the resistance of distilled water (in a clean glass).
The world around you is full of materials that conduct electricity with varying amounts of resistance.
Cleanup and Recycling
Your battery should not have been damaged or significantly discharged by this experiment. You’ll be able to use it again.
Remember to switch off your meter before putting it away.
Experiment 2: Let’s Abuse a Battery!
To get a better feeling for electrical power, you’re going to do what most books tell you not to do. You’re going to short out a battery. A short circuit is a direct connection between the two sides of a power source.
 Short Circuits
Short Circuits
Short circuits can be dangerous.
Do not
short out a power outlet in your home: there’ll be a loud bang, a bright flash, and the wire or tool that you use will be partially melted, while flying particles of melted metal can burn you or blind you.
If you short out a car battery, the flow of current is so huge that the battery might even explode, drenching you in acid (Figure 1-30).
Lithium batteries are also dangerous. Never short-circuit a lithium battery: it can catch fire and burn you (Figure 1-31).
Use only an alkaline battery in this experiment, and only a single AA cell (Figure 1-32). You should also wear safety glasses in case you happen to have a defective battery.
You will need:
- 1.5-volt AA battery
- Single-battery carrier
- 3-amp fuse
- Safety glasses (regular eyeglasses or sunglasses will do)
- Alligator clip (small or large)
Figure 1-30.
Anyone who has dropped an adjustable wrench across the bare terminals of a car battery will tell you that short circuits can be dramatic at a “mere” 12 volts, if the battery is big enough.
Figure 1-31.
The low internal resistance of lithium batteries (which are often used in laptop computers) allows high currents to flow, with unexpected results. Never fool around with lithium batteries!
Figure 1-32.
Shorting out an alkaline battery can be safe if you follow the directions precisely. Even so, the battery is liable to become too hot to touch comfortably. Don’t try this with any type of rechargeable battery.
Procedure
Use an alkaline battery. Do not use any kind of rechargeable battery.
Put the battery into a battery holder that’s designed for a single battery and has two thin insulated wires emerging from it, as shown in Figure 1-32. Do not use any other kind of battery holder.
Use an alligator clip to connect the bare ends of the wires, as shown in Figure 1-32. There will be no spark, because you are using only 1.5 volts. Wait one minute, and you’ll find that the wires are getting hot. Wait another minute, and the battery, too, will be hot.
The heat is caused by electricity flowing through the wires and through the electrolyte (the conductive fluid) inside the battery. If you’ve ever used a hand pump to force air into a bicycle tire, you know that the pump gets warm. Electricity behaves in much the same way. You can imagine the electricity being composed of particles (electrons) that make the wire hot as they push through it. This isn’t a perfect analogy, but it’s close enough for our purposes.
Chemical reactions inside the battery create electrical pressure. The correct name for this pressure is
voltage
, which is measured in volts and is named after Alessandro Volta, an electrical pioneer.
Going back to the water analogy: the height of the water in a tank is proportionate to the pressure of the water, and comparable to voltage. Figure 1-33 may help you to visualize this.
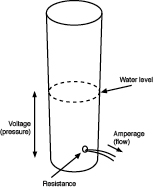
Figure 1-33.
Think of voltage as pressure, and amperes as flow.
