Criminal Poisoning: Investigational Guide for Law Enforcement, Toxicologists, Forensic Scientists, and Attorneys (21 page)
Authors: John H. Trestrail

113
Figure App.-1
standing in a snowfield. The retinal toxicity is caused by the accumulation of formic acid.
•
Ethylene glycol: In the first few hours, the victim will appear inebriated.
Gastritis and vomiting may occur. After a period of 4–12 h, the patient will experience acidosis, hyperventilation, convulsions, coma, and cardiac conduction disturbances and arrhythmias. Kidney failure is common.
Symptoms—Chronic:
For both types of antifreeze, chronic administration is less likely. The symptoms, however, would be the same, with a more gradual onset.
Disease Confusion:
•
Methanol: Uremia, diabetic ketoacidosis, and alcoholic ketoacidosis.
•
Ethylene glycol: Diabetic ketoacidosis and lactic acidosis.
Victim Notes:
None.
Detection of Antifreeze
Specimens:
For both types of antifreeze, beverages, liquid medications, and blood; and in the deceased, vitreous humor (from the eye).
Method:
•
Methanol: Gas chromatography (GC), the same technique as used for ethanol determination. An elevated anion gap acidosis in the patient supports 114
Common Homicidal Poisons
the diagnosis. NOTE: Enzymatic methods, sometimes used for ethanol determinations, do
not
readily detect methanol.
•
Ethylene glycol: GC. NOTE: It has been observed that the concentration of ethylene glycol in postmortem tissue may rapidly decline during refrigera-tion if a colorimetric method for analysis is used. A high osmolar gap in the patient is often indicative of intoxication.
Toxic Levels (fatal cases):
(1)
•
Methanol
Units
Blood
Brain
Liver
Kidney
Urine
mg/L
400
?
?
?
?
Range (mg/L)
200–6300
?
?
?
?
•
Ethylene glycol
Units
Blood
Brain
Liver
Kidney
Urine
g/L or g/kg
2.4
2.0
6.7
4.6
5.7
Range (g/L
0.3–4.3
0.3–3.9
0.2–15.1
0.2–11.3
0.6–10.8
or g/kg)
Analysis Notes:
None.
Selected Antifreeze Homicide Cases
Bobbie Jan Nichols (1992)
Angelina Rodriguez (2000)
Julia Lynn Turner (2001)
Sandra Kay Baker (2003)
ARSENIC
Form:
Metallic arsenic (As) is a steel-gray, brittle metal. Arsenic trichloride (AsCl3) is an oily liquid. Arsenic trioxide (As2O3) is a crystalline solid and it can also exist as arsine gas (AsH3).
Lewisite
, a gas used as a weapon in war, is a derivative of arsine.
Color:
Metal, steel gray; salts, white.
Odor:
Odorless, but arsenic can produce a garlicky odor to the breath.
Solubility:
Arsenical salts are water soluble.
Taste:
Almost tasteless.
Source:
Pesticides, rodent poison, ant poison, homeopathic medications, weed killers, marine (copper arsenate) and other paints, ceramics, livestock feed.
Lethal Dose:
Acute, 200 mg (As2O3); chronic, unknown.

Appendix
115
Figure App.-2
How It Kills:
Arsenic is a general protoplasmic poison; it combines with sulfhydryl (—SH) groups on enzymes to inhibit their normal function.
This inhibition results in disruption of normal metabolic pathways related to energy transfer.
(
See
Fig. App. 2
.)
Poison Notes:
The trivalent arsenic (As+3) is more toxic than the pen-tavalent (As+5) form. Arsenic is one of the oldest poisons used by humans.
Victim of Arsenic
Administered:
Often administered to victim in food or drink.
Symptom Onset Time Interval:
Hours to days.
Symptoms—Acute:
Gastrointestinal (GI) (30 min to 2 h postexposure): vomiting, bloody diarrhea, severe abdominal pain, burning esophageal pain, metallic taste in the mouth. Later symptoms include jaundice, kidney failure, and peripheral neuropathies (destruction of the nervous system). Death from circulatory failure can occur within 24 h to 4 d.
Symptoms—Chronic:
GI (diarrhea, abdominal pain), skin (hyperpig-mentation of palms and soles), nervous system (symmetrical sensory neuropathy with numbness and loss of vibratory or positional sense, burning pain on the soles of the feet), other localized edema (face, ankles), sore throat, stomatitis (mouth inflammation), pruritis (itching), cough, tearing, salivation, garlic odor on breath, Aldrich-Mees lines (horizontal white lines that nor-116
Common Homicidal Poisons
mally take 5 to 6 wk to appear after the exposed nail bed area grows), hair loss.
Disease Confusion:
Gastroenteritis, neurological disease.
Victim Notes:
In homicides the amount of arsenic could be administered in a single, large acute dose or in frequent, small chronic doses to make the symptoms appear like those of a progressing natural illness. In suicides, the amount of arsenic taken is usually large.
Detection of Arsenic
Specimens:
Food, beverages, medications, blood, urine, gastric contents, hair, nails, autopsy organ specimens.
Method:
Colorimetric, atomic absorption.
Toxic Levels:
(1)
Blood
Urine
Gastric
Other
0.6–9.3 mg/L
3300 µg/L
Unknown
3 ppm (hair/nails)
>1 µg/g dry weight
Analysis Notes:
Arsenic can be detected in hair and bones many years after poisoning. Several hairs pulled out by the root should be sent for analysis with a clear indication of which end of the hair is the root. Segmented hair analysis using neutron activation gives an indication of arsenic exposure over the last several months.
Selected Arsenic Homicide Cases
Mary Blandy (1752)
Madeline Smith (1857)
Florence Maybrick (1889)
Johann Hoch (1905)
Henry Seddon (1911)
Mabel Greenwood (1919)
Herbert Armstrong (1921)
Michael Swango (1985)
Marie Hilley (1986)
Blanche Moore (1988)
BOTULINUS TOXIN
Form:
Usually in the liquid form from culture medium.
Color:
Colorless.
Odor:
Odorless.

Appendix
117
Figure App.-3
Solubility:
Water soluble.
Taste:
Tasteless.
Source:
Produced by the bacteria
Clostridium botulinum
.
Lethal Dose:
Acute, 50 ng; chronic, not applicable. Estimated to be as little as 0.1 mL of contaminated food. This substance is the most toxic known.
The toxin is 7,000,000 times more lethal than cobra venom.
How It Kills:
Botulinus toxin irreversibly binds to cholinergic nerve terminals and prevents the release of acetylcholine from the axon. Severe muscle weakness results, and subsequently death from respiratory failure.
Poison Notes:
The toxin can grow in home-canned food at pH > 4.5.
Victim of Botulinus Toxin
Administered:
Can be administered in cool food or drink. Heating to a boiling temperature destroys the toxin within a few minutes.
Symptom Onset Time Interval:
May be slow to onset (2 h to 14 d).
Death may occur as early as 10 h after the symptoms first appear.
Symptoms—Acute:
Dry, sore throat; dry mouth; dizziness; vomiting; stomach upset; difficulty in swallowing; difficulty in speaking; double vision; drooping eyelids; cranial nerve weakness; progressive symmetric descending paralysis; and respiratory arrest.
Symptoms—Chronic:
Not applicable.
118
Common Homicidal Poisons
Disease Confusion:
Viral illness, Guillain-Barré syndrome, stroke, tick paralysis, heavy-metal poisoning, adverse drug reactions, and many other conditions.
Victim Notes:
None.
Detection of Botulinus Toxin
Specimens:
Diagnosis is confirmed by determination of the toxin in serum, stool, or a wound; food; beverages; medications; blood; urine; and gastric contents. The test may be negative if the samples were collected late or the quantity of the toxin is small.
Method:
Analysis is usually carried out by local health departments, or the Centers for Disease Control in Atlanta, Georgia.
Toxic Levels:
(1)
Blood
Urine
Gastric
Other
Not applicable
Not applicable
Not applicable
Not applicable
Analysis Notes:
None
Selected Botulinus Toxin Homicide Cases
No cases on record.
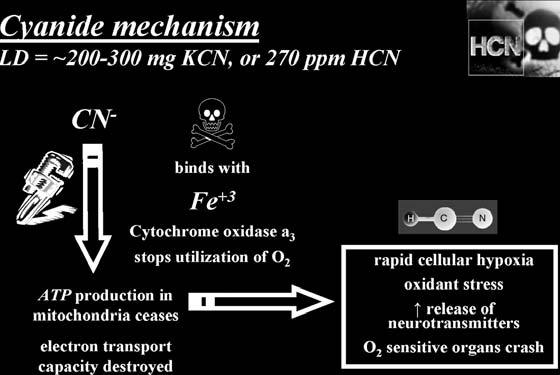
CYANIDE
Form:
In liquid form, hydrogen cyanide (HCN) is also known as prus-sic acid. Pure hydrogen cyanide is a gas usually made by mixing an acid with cyanide salts. It is more commonly found in the form of sodium, potassium, or calcium salts, which are crystalline materials. Industrial cyanide can take the form of large nuggets called “cyanide eggs.”
Color:
Sodium and potassium salts of HCN are white.
Odor:
It is supposed to elicit the odor of almonds. However, 40–60% of the population cannot detect the odor because of a genetic inability—a form of “odor blindness.”
Solubility:
Salts of cyanide are easily dissolved in aqueous liquids. Acidic liquids would cause the release of some HCN gas.
Taste:
Salts of cyanide have a bitter (alkaline) taste and can have a mild corrosive action on tissues.
Source:
Fumigants, insecticides, rodenticides, metal polishes (especially silver polish), electroplating solutions, metallurgy for the extraction of gold and silver from ore, photographic processing, jewelers, and chemical labora-
Appendix
119
Figure App.-4
tories. Cyanide is contained as cyanogenic glycosides inside the pits and seeds of certain plants of the genus
Prunus
(e.g., cherry, peach, almond, cassava, and apricot). Cyanide can also be produced by the action of a flame on the synthetic plastic materials polyurethane and polyacrylonitrile. In addition, it is found as part of the intravenous antihypertensive drug molecule sodium nitroprusside (Nipride®). Cyanide is used in the most commonly employed method of synthesizing phencyclidine (PCP).
Lethal Dose:
Acute, 270 ppm (air), 50 mg (HCN), 200–300 mg (NaCN
or KCN); chronic, unknown in all forms.
How It Kills:
Cyanide shuts down respiration at the cellular level by inactivating essential enzymes, resulting in metabolic asphyxiation. Critical effects are on those organs most sensitive to oxygen deprivation, the brain and heart.
