Bad Pharma: How Drug Companies Mislead Doctors and Harm Patients (47 page)
Read Bad Pharma: How Drug Companies Mislead Doctors and Harm Patients Online
Authors: Ben Goldacre

- All doctors should declare all payments, gifts, hospitality, free teaching and so on, to their patients, to colleagues, and to a central register. The conventional cut-off is for everything within the past three years, but we could consider making it longer. We should display the contents in our clinics, to our patients, and let them decide if such activities are acceptable.
- Drug companies should declare all payments to doctors to a central database, naming each doctor, and giving the amount paid to them, and what it was for. This will permit cross-checking and make declarations easier.
- Governments should create a publicly accessible national database of payments by companies to doctors, and make it compulsory for doctors and companies to declare everything to it. Until they do, someone else could make a voluntary one.
- The US Sunshine Act is a good starting model for legislation: companies will be compelled to declare who they gave money to, how much, and on what date; but also what drug the payment related to. This information, displayed by doctors in waiting rooms, would be just fine.
- Conflict-of-interest policies vary hugely between institutions, and have never been reviewed in the UK. In the US this work has been done by the American Medical Students Association. Its website, www.amsascorecard.org, is a model to us all: it grades over a hundred institutions on their conflict-of-interest policies for gifts, consulting, speaking, disclosure, samples, drug reps, industry support for education and so on, using a transparent methodology and giving a summary grade for each institution, from A to F. Honestly, I feel weepy when I look at it.
You may be feeling overwhelmed, and I couldn’t blame you. We should take a moment to recap, to think about how an industry executive would defend themselves, and then work out how to fix things.
For me, missing data is the key to this whole story. Bad behaviour in marketing departments is unpleasant, but it’s the one thing that has already received public condemnation, because the issues are tangible, with covert payments, misleading messages, and practices that are obviously dishonest, even to the untrained eye. But for all that they may be disappointing, these distortions can be overcome by any good doctor. If you go straight to the real evidence, and read systematic reviews of good-quality trials, then all the distortions and spin of drug reps and ‘key opinion leaders’ are nothing more than wasteful, irrelevant noise.
Missing data is different, because it poisons the well for everybody. If proper trials are never done, if trials with negative results are withheld, then we simply cannot know the true effects of the treatments that we use. Nobody can work around this, and there is no expert doctor with special access to a secret stash of evidence. With missing data, we are all in it together, and we are all misled. I will say this only once more, but I think it bears repeating: evidence in medicine is not an abstract academic preoccupation. Evidence is used to make real-world decisions, and when we are fed bad data, we make the wrong decision, inflicting unnecessary pain and suffering, and death, on people just like us.
In a moment, we will look at what can be done: because there are some simple fixes that would put all this behind us, and hugely improve patient care, globally, at almost no cost, if patients and politicians were willing to fight for them. But before that, I’d like to look at what the pharmaceutical industry will say in response to this book.
Firstly, I’m sure – perhaps after some dismissive personal smears – there will be accusations of cherry-picking. People will claim, incorrectly, that I have focused on rare and exceptional cases. On this, I would encourage you to remember how much of this book is based on systematic reviews, which summarise all of the evidence ever collected on a given question. Go back and check, if you like. Our best estimate was that half of all clinical trials go unpublished, and that doesn’t come from a story, or an anecdote: it comes from the most current systematic review, containing the results of every study ever conducted on this issue. Where we have walked through individual, shameful cases – like paroxetine, or Tamiflu, or Orlistat – it was only to put narrative meat on these very ugly bones.
So I am confident that you will agree, from the evidence set out in this book, that these are systemic problems; and that it would be shameful, or even dishonest, simply to dismiss them. What’s more, where the evidence is lacking – and this isn’t often – I have been clear, and I have set out what work is needed to fill those gaps. For example: lectures from key opinion leaders paid by industry are one of the most significant ways in which qualified doctors are educated today, and two decades ago ‘mystery shopper’ research found that these lectures are systematically biased. The fact that this work hasn’t been repeated in the past five years should be a source of shame for the industry and for my profession. It’s not a cause for celebration, and it certainly doesn’t exonerate anyone.
As their next tactic, we can be sure – because we’ve watched them do this already – that people from industry will point to their guidelines. Look at all these miles and kilograms of rules, these vast offices filled with regulators: this is one of the most closely monitored industries in the world, they will say, drowning in red tape. But we have proved, I think, that these regulations simply do not do their job. The rules on registering trials were ignored; the FDA rules on posting results within a year have only been obeyed for a fifth of trials; the ICMJE regulations on ghostwriting – absurdly – permit ghostwriting; and so on. These regulations have been tested, and they have been shown to fail.
But the most dangerous tactic of all is the industry’s enduring claim that these problems are all in the past. This is deeply harmful because it repeats the insult of all the fake fixes we have seen throughout this book: and it is this recurring pattern of flat denial that allows the problems to persist.
The clearest window onto this strategy comes from the industry’s response to its most recent public scandal. In July 2012, GSK received a $3 billion fine for civil and criminal fraud, after pleading guilty to a vast range of charges around unlawful promotion of prescription drugs, and failure to report safety data. The full list of charges and evidence is vast – you can browse it all at the Department of Justice website – but the methods they used will be very familiar to you by now.
GSK bribed doctors with gifts and hospitality; it paid doctors millions of dollars to attend meetings, and to speak at them, in lavish resorts; it used, in the justice department’s own words, ‘sales representatives, sham advisory boards, and supposedly independent Continuing Medical Education (CME) programs’. It withheld data on the antidepressant paroxetine. It engaged in off-label promotion and kickbacks for the asthma drug Advair, the epilepsy drug Lamictal, the nausea drug Zofran, Wellbutrin, and many more. On top of all this, it made false and misleading claims about the safety profile of its diabetes drug rosiglitazone; it sponsored educational programmes suggesting there were cardiovascular benefits from the drug, when in reality even the FDA label said there were cardiovascular risks; and most damningly of all, between 2001 and 2007, it withheld safety data on rosiglitazone from the FDA.
1
Industry spokespeople first said that these issues did not relate to practice in the UK. But that is not true. GSK is a UK company, with a UK head. In ‘Exhibit Six’ of the US court documents, you will find a collection of media stories, presented because they arise from off-label promotion of GSK’s drugs. The first story is a eulogy for the anti-smoking drug Zyban, taken from the
Guardian
, a UK newspaper, and written by Dr Roger Henderson, a UK GP who writes widely in UK newspapers (visiting his website today, I see that as well as doing journalism he also advertises his services as a PR consultant to the pharmaceutical industry).
The Times
, another UK newspaper, comes next in this bundle of evidence, with the headline ‘Now
That
is a Wonder Drug: Can One Pill Ease Depression, Help You Lose Weight, and Stop You Smoking?’. The
Daily Mail
asks: ‘Is This Anti-Depressant a New Weight-Loss Drug?’. The
Sun
carries a similar story. A large part of the GSK fraud relates to the mis-selling of paroxetine, which was also, you will remember, the subject of a four-year-long investigation in the UK.
These acts were perpetrated on our soil, and if they weren’t detected here, that’s partly because we weren’t trying very hard: it’s a little-known fact in the UK, but in the US, corporate whistleblowers get a cut of any fines that are levied. This is a policy designed to incentivise people to come forward with evidence of corporate crime, and it’s reasonably effective. A small group of whistleblowers in the GSK case will now share roughly $600 million between them. In the UK, whistleblowers are sacked and silenced.
But that wasn’t the only tactic. Next, the industry claimed that these crimes were all in the past. GSK’s own press release said they were from a ‘different era’. Stephen Whitehead, the head of the ABPI (who previously worked in PR for GSK, Barclays and the alcohol industry), said: ‘The global pharmaceutical community has fundamentally changed during recent years; where we have made mistakes in the past, we have tried to rectify them.’
To examine this claim – even if we set aside the vast amount of evidence in this book – it’s useful to trace the current positions of the people who were in senior roles at GSK, during the period of proven fraudulent behaviour, and then look at where they are now. Chris Viehbacher of GSK was singled out in the court ruling: he is now CEO of Sanofi, the third-biggest drug company in Europe. Jean Pierre Garnier was CEO of GSK from 2000 until 2008, only four years ago: he is now chairman of Actelion, a Swiss pharmaceutical company.
2
There is no suggestion that these companies have been involved in improper behaviour. The court also specifically mentioned Lafmin Morgan, who worked at GSK in marketing and sales for twenty years: Morgan was still working for GSK in 2010, just two years ago.
3
So while GSK and the ABPI may claim that these problems are in the past, in reality: one of the charges involves withholding safety data as recently as 2007, on a drug only suspended from the market in 2010; two of the most senior figures pulled out in the court case are at the helm of pharmaceutical companies in Europe right now; and another senior figure in marketing at GSK continued to work for the company until just two years ago.
It doesn’t end there. Richard Sykes was the head of GlaxoWellcome from 1995 to 2000, and then chair of GSK from 2000 to 2002, when many of these fraudulent acts took place. He is now the chair of Imperial College Healthcare NHS Trust, and chair of the Royal Institution in London, the UK’s oldest and most eminent science communication establishment. That, more than anything, is a clear illustration of the extent to which this world penetrates British academia and medicine to its absolute core.
To be clear, Richard Sykes is not the only example, and I have been extremely restrained about naming medics on the industry payroll, not out of kindness or loyalty, but for the simple reason that once you start, stopping becomes arbitrary. John Bell, Oxford Professor of Medicine, the President of the Academy of Medical Sciences, is on the board of Roche, which is still withholding information on Tamiflu, as you have seen. Mark Porter, from
Case Notes with Mark Porter
on BBC Radio 4, was paid by Eli Lilly to present their ‘disease awareness campaign’ videos around Cialis. These are meaningless, banal, lucky-dip examples: ignore them, forget their names, because they are the norm.
And contrary to the spin, this GSK fine was no isolated incident either. Eli Lilly was fined $1.4 billion in 2009 over its off-label promotion of the schizophrenia drug olanzapine (the US government says the company ‘trained their sales force to disregard the law’). Pfizer was fined $2.3 billion for promoting the painkiller Bextra, later taken off the market over safety concerns, at dangerously high doses (misbranding it with ‘the intent to defraud or mislead’). Abbott was fined $1.5 billion in May 2012, over the illegal promotion of Depakote to manage aggression in elderly people. Merck was fined $1 billion in 2011. AstraZeneca was fined $520 million in 2010.
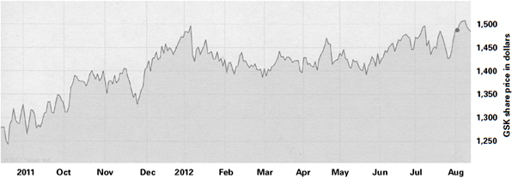
These are vast sums of money: Pfizer’s in 2009 was the largest criminal fine ever imposed in the US, until it was beaten by GSK. But when you consider these figures alongside the revenue for the same companies, it becomes clear that they are nothing more than parking tickets. For the period of time covered by the $3 billion GSK settlement, sales of rosiglitazone were $10 billion, paroxetine brought in $12 billion, Wellbutrin $6 billion, and so on.
4
Here is a graph of GSK’s share price over the past year: decide for yourself if you can see any impact from a $3 billion fine and criminal fraud case in July 2012.