Read A New History of Life Online
Authors: Peter Ward
A New History of Life (11 page)
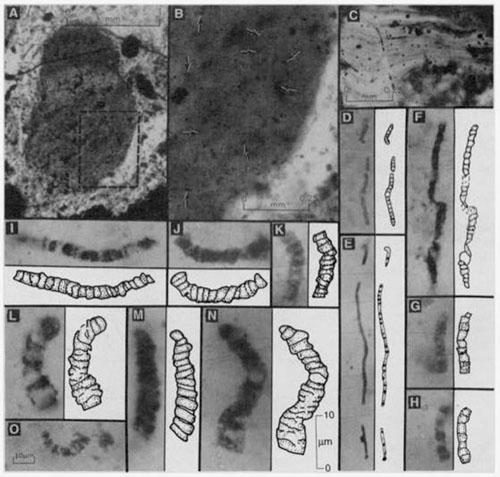
The famous images of the supposedly oldest record of fossil life, as published by Bill Schopf of UCLA in the 1980s and 1990s. These fossils were then dated at over 3.5 billion years in age. Subsequently both that age date (now thought to be a billion years younger) and even their identification as fossils is under attack.
Major changes affected the Earth and the history of life around 2.5 billion years ago—changes so consequential that it marks a new era on the hierarchical geological time scale. The oldest era is the Hadean, which began with the Earth’s formation (4.567 billion years ago) and ended with the appearance of the first rock record, at about 4.2 billion years ago. The Archean era succeeded it, a violent time in Earth history, which began with the start of the heavy bombardment and ended ~2.5 million years ago, with the succeeding era named the Proterozoic. The changeover from the Archean to the Proterozoic coincided roughly with the rise in oxygen—and that oxygen was created by photosynthetic life.
Photosynthesis is the process that life uses to turn inert carbon dioxide into living cell matter (and thus changes inorganic carbon to what we call organic carbon). There is evidence that some kind of photosynthetic organism was present during the Archean, the time 4.2 to 2.5 billion years ago when life first evolved. It also seems clear that the evolution of photosynthesis postdated the oldest life. The first life probably used hydrogen in a compound in which the hydrogen is chemically merged with a sulfur atom, producing the very important (to the history of life) compound called hydrogen sulfide for its energy needs.
1
Hydrogen is energy rich, which is why human technology is trying to harness it for everything from cars to power plants. We also do know that the Archean organisms appear to have used the major life-required elements still used by life today: carbon, sulfur, oxygen, hydrogen, and nitrogen.
We have some information on the nature of the oceans and atmosphere at 3.5 billion years ago. Carbon dioxide was probably at much higher concentration than we see today. There was probably a great deal of water vapor in the atmosphere, as well as the gas methane, the kind of atmosphere that holds in heat, thus warming the planet at a
time when the sun was far less energetic. Without those Archean greenhouse gases—water vapor, methane, and carbon dioxide—there would have been no liquid water on the planet. Greenhouse gases create a warming mechanism that the planet otherwise would not have, an atmosphere that could trap heat. But it was also an atmosphere without oxygen.
Much of our understanding of life during this long Archean chapter comes from studying modern-day environments that appear to be useful analogues. Low-oxygen environments are relatively rare in our oceans today, but they’re much more common in smaller lakes. In fact, many modern-day lakes are essentially stratified, with a thin layer of oxygen (absorbed out of air), underlain by water that has no oxygen at all. The study of the microbial communities living in these types of environments has provided insights into what life must have been like in the deep past. One of the important groups of organisms that are necessary for carbon cycling in the modern-day lakes, and most likely in the ancient waters of the Archean ocean, is associated with the chemical methane. As noted earlier, methane gas helps trap heat reflected off the Earth by sunlight from escaping into space.
2
Some bacteria can break down methane and use it for food. Much of the Earth’s early life used methane in this way, telling us that soon after life evolved on Earth, it quickly diversified in the ways that it acquired energy, just as the evolution of the car evolved along lines in which cars get energy—first from steam, then diesel fuel, then gasoline (both diesel and gasoline are carbon compounds that contain energy, just as methane is), and soon hydrogen fuel. While our civilization comes last to this fuel source, life came to it first.
Much of the evidence telling us about the history of early life on Earth comes from the sedimentary rock record. For instance, one of the characteristics of Archean sedimentary deposits is the frequent appearance of brightly colored red layers within some Archean-aged sedimentary rocks. These are called banded iron formations, or BIFs, and these interesting sedimentary rocks have not been produced on the Earth’s surface in any sort of significant accumulation for the past 1.85 billion years, except during one or two snowball Earth intervals at
the end of Precambrian time, which we will describe in far greater detail in the next chapter.
There is a long-standing puzzle associated with these BIF sediments—in order to be distributed so widely and gently, the iron has to have been dissolved in water—and that means it should have been in the greenish reducing form called
ferrous iron
. On the other hand, for it to have been precipitated out means that it was rusted into the red
ferric
form, which is not soluble in water at all: it simply falls out of water as particles, rather than dissolving in water, as a cube of sugar would. The problem is oxygen: ferrous iron reacts instantly with free molecular O
2
to form the red ferric state. Any iron or iron mineral that is bright red in color tells us that the iron has undergone this chemical change, which we commonly call rust, and that almost always requires molecular O
2
. How could oxygen levels in the ocean waters be low enough to allow the iron to stay in the green soluble form, and yet then be available to make it rust? This was a long and perplexing scientific mystery.
Over fifty years ago, one of the important figures in Precambrian paleobiology, Preston Cloud of the University of California at Santa Barbara, hypothesized that the oxygen needed to turn dissolved ferrous iron into the rusted, particular ferric iron in the oceans came from a group of primitive photosynthetic microbes known as the blue-green algae, which are now called the cyanobacteria.
3
This is the only organism on Earth that ever learned how to perform the life-giving process of oxygenic photosynthesis, which is literally the ability to cleave a water molecule and liberate its oxygen atom. Some of their descendants were enslaved by other organisms, and now serve us all as the green light-gathering organelles in plants and other algae. Every plant on Earth now has tiny “capsules” that evolved from those first cyanobacteria, but are now “endosymbiosis” slaves doing the bidding of the multicellular plant. Preston Cloud envisioned a floating “oxygen oasis” of these first tiny photosynthesizers, the cyanobacteria, each excreting tiny amounts of oxygen, and over hundreds of millions of years radically changing the nature of not only life on Earth, but the chemistry of our planet’s oceans, atmosphere, and even rock cover. With each
tiny trace of new oxygen liberated into the ancient Archean sea, tiny flakes of rust would then settle to the ocean bottom, slowly but inexorably accumulating to make the banded iron formations.
Molecular oxygen is one of the most toxic compounds around. Anyone who takes antioxidants along with their vitamin supplements knows that they fight cancer—and cancer is usually caused by oxygen wrecking delicate cell chemistry at the wrong time, in the wrong place, and changing it into a new zombie-like killer cell as a result. Antioxidants are not just an advertising slogan. Oxygen is a cell wrecker, cell changer, and often cell killer because of its chemical ferociousness. So how could the organisms producing this poison survive as soon as the oxygen molecules were liberated?
This now leads to a classic “chicken and the egg” problem: any early life form that evolved a system to release O
2
without having protective antioxidant enzymes would have killed itself, so the systems to control oxygen would have had to evolve first. But all of the oxygen in our atmosphere is produced by oxygenic photosynthesis, so there should not have been any oxygen before this to drive the evolution of the protective enzymes! Thus, there must have been some nonbiological source that produced trace amounts of molecular oxygen and then exposed primitive cells to it in an environment where they could gradually evolve enzyme systems to protect them from this poison, in a way analogous to how we protect ourselves from killer diseases by exposing ourselves to tiny amounts of the disease when we are very young, letting our body gradually build up defenses.
But where did this early oxygen “vaccine” come from if not from photosynthesis? It is very difficult to produce oxygen in nonbiological ways, but one way that works is through photochemical reactions involving ultraviolet radiation, the same UV that causes sunburns on unprotected skin. UV radiation hitting water and CO
2
molecules in the atmosphere will generate trace levels of O
2
and other chemicals. Today, solar UV radiation is mostly blocked by a layer of ozone high in the atmosphere, far above the layers with water vapor (that freezes out). But early in Earth history there was no oxygen and thus no ozone, and hence no UV screen. Thus very strong ultraviolent radiation from the
sun blasted the Earth, creating a tiny number of oxygen molecules. Unfortunately, reactions similar to those that generate the oxygen quickly snuff it out, making it unlikely to survive long enough to generate a biological effect, particularly as this is all happening in a UV radiation bath that is very good at scrambling DNA and sterilizing anything it hits. What is needed is a mechanism that allows the oxygen to be separated from the other products (hydrogen and CO in particular) before it is snuffed out.
Two processes are known that can do this. First, if water gets high up in the atmosphere, a significant fraction of the hydrogen atoms liberated from the UV light will be traveling faster than Earth’s escape velocity and can be lost to space. That will leave a small trickle of oxygen, ozone, and hydrogen peroxide diffusing down from above (which are too heavy to escape), but it is really only a trickle. Reducing gases produced by biological activity and from volcanic eruption will easily squelch these oxic compounds long before they could reach the biosphere. The second process occurs on the Earth’s surface—but on the surface of a glacier! In Antarctica today the “ozone hole” permits a wider spectrum of UV radiation to reach the surface, where it can blast water molecules apart, eventually generating H
2
gas and H
2
O
2
(hydrogen peroxide). That peroxide gets locked in the ice, separated from the H
2
gas. Working with a graduate student at Caltech, Danny Liang, we calculated that up to 0.1 percent of the ice during a Precambrian glaciation could be made of H
2
O
2
, which, when the glacier melts, would be converted into O
2
and water.
4
Although this is not enough to breathe, it is enough to cause life with its powerful tool kit we call evolution to react. As noted below, we think the first oxygen-releasing cyanobacterium evolved during one of these Precambrian glacial intervals, and it certainly must have had evolved protection from oxygen.
In a 2008 paper, one of the most experienced researchers about life and the early Earth, Roger Buick of the University of Washington, broke down the alternatives to the “when” of oxygenation as follows: First, oxygenic photosynthesis (such as is common today in all green plants) evolved hundreds of millions of years before the atmosphere
became significantly oxygenated, because it took eons to oxidize the continued production of reduced volcanic gases, hydrothermal fluids, and crustal minerals. Second, it arose at ~2.4 billion years ago in what we refer to in these pages as the great oxidation event, causing immediate environmental change. Third, oxygen production from photosynthesis or any other means began very early in Earth’s history, before the start of the geological record, leading to an Archean (greater than 2.5GA) atmosphere that was highly oxygenated. To choose between these alternatives, let’s look at the record as we now know it, because this is so critical to a good understanding of the history of life, and indeed there is a great deal that is “new” about this history in terms of our knowledge.
Despite the widespread agreement that the evolution of the cyanobacteria was the most profound biological event on this planet (even more so than the evolution of the eukaryotic cell, and then multicellular life), there is a surprising disagreement about exactly when this seminal biological innovation happened. Over fifty years ago, geologists realized that some of the oldest stream-deposited sedimentary rocks on Earth contained rounded grains of the common mineral called pyrite (fool’s gold) as well as another mineral containing tiny amounts of uranium (the mineral named uraninite). These minerals are extremely unstable in the presence of oxygen (like iron, they quickly rust), and are never found in our open, oxygenated oceans and land areas unless completely cut off from contact with our normal, oxygenated atmosphere. That led to the initial concept that the atmosphere contained very little oxygen until some time near the end of Archean time, perhaps as late as 2.5 billion years ago or even later. Most of the geological community agrees that even at these dates, oxygen concentrations in the atmosphere were so low that both pyrite and uraninite grains could exist on land and in the sea without rusting, and in fact in rocks created as late as 2.5 billion years ago we find both pyrite and
uraninite in abundance, telling us that at those dates the amount of oxygen in air and sea would have been nil. Yet by 2.4 billion years ago both kinds of minerals disappear from rocks created underwater or on land. Does this mean that cyanobacteria thus evolved only after 2.5 to 2.4 billion years ago? This has spurred a profound debate of great importance to understanding the history of life.

